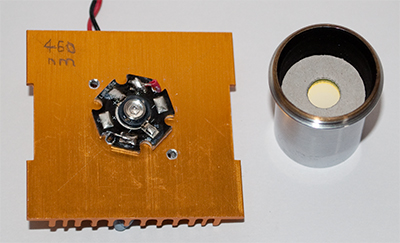
Forays into Fluorescence - 5
Notes on adapting a Zeiss Photomicroscope (or Universal) to LED sourced epi-fluorescence
by David Walker, UK
(The basic approach may also suit Zeiss Standards with epi fluorescence heads.)
The author as a hobbyist began to explore fluorescence microscopy two years ago and aspects of the technique that appealed while on a very steep learning curve have been presented in earlier 'Forays'. Initially I jointly acquired with my brother a Leitz Diaplan with epi-fluorescence Ploemopak head and a 100W mercury lamp. This gave a very useful insight into the capabilities of a correctly setup fluorescence microscope for both visual and photographic studies. In
parallel, I began to adapt my Zeiss Photomicroscope for both transmitted and epi fluorescence using just the normal 100W halogen lamp, to assess the limitations of the simpler, but cheaper and safer setup.
As other hobbyists have found, and remarked on by Laurent Delvoye in his Micscape article 'Fluorescence Microscopy with Super LEDs', there are major disadvantages on running a mercury lamp in a domestic environment (see Appendix) and decided to sell the Diaplan setup and build up the Photomicroscope for fluorescence work (space considerations were also a criteria).
The author has yet to explore the use of fluorochromes as has found autofluorescence of a variety of subjects so fascinating. See earlier 'Foray' articles for notes on autofluorescence of chlorophyll containing plant structures (epi and transmitted), various dry mounts and snail radulae.
The exciting wavelength found most useful for the autofluorescence was a deep blue. The normal 100W quartz halogen bulb has been used to date for this with Zeiss IIIRS head and default 'blue excitation' setup. The main disadvantage cf a mercury lamp being that the visual image can be quite dull and a consumer digital SLR camera is relied on for studying the subjects (from photo trials a 100W halogen lamp was ca. 3-4 camera stops duller than a mercury
lamp for deep blue).
Safety note: Power LEDs are extremely bright; even for LEDs emitting at visible wavelengths, viewing a spot of light of this intensity may do eye damage. Deep blue as well as near UV is reported to be dangerous to the eyes (1). On a microscope with mercury lamps, safety is assured with the correct dichromatic mirrors and excitation / blocking filters
for a given wavelength. But LEDs may not necessarily match the original mirror / filter combination, so if experimenting with none standard combinations, extra care needs to be taken. The author would not use LEDs of this intensity for transmitted studies.
Set-up
The LED unit was adapted from Laurent Delvoye's neat design of plug-in modules for a Leitz lamphouse where the LED was attached to a CPU heatsink and which directly replaced the lamp. The present author adopted a simpler design to fit directly onto the Zeiss epi side tube, as didn't have a spare lamphouse to dedicate to fluorescence. The design is illustrated and described below.
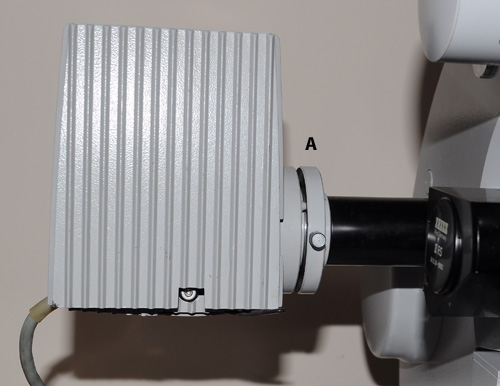
The author's usual epi-fluorescence setup using a 100W quartz halogen bulb in lamphouse attached to the Zeiss Photomicroscope epi side tube. The plate A is attached by three screws and was removed for the LED trials below. There is a fixed internal stop (ca. 1 cm diameter) with lens in the epi tube ca. 6 cm from plate A. The Zeiss III RS can be seen towards the front right, the author rebuilt a 'cooked' example of a III RS
head, described in an earlier article.
The Zeiss epi side tube is a quite good value accessory and sits in the epi port on limb of the scope. This prevents the use of the epi condenser however, although many epi studies use the full NA of the objective. The dedicated epi-condenser for the Photomicroscope (not the same as that for the Universal) seems a hard to source accessory, this allows a lamp at rear to be used for either transmitted or incident. Although that
setup would need great care with the intensely bright LEDs, especially if deep blue or UV sources are used.
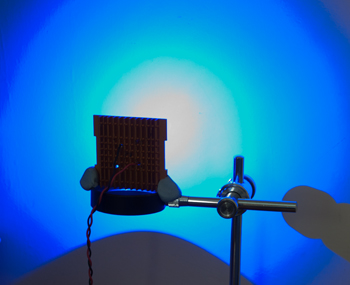
Left above: Power LEDs typically have a Lambertian light emission (160º 'viewing angle' for this example)*; when shone at a wall the same distance away as the LED would be from the fixed relay lens / stop in Zeiss epi tube, there is a central beam but too wide and much light is wasted. (* A 'batwing' and 'side emitter' is also common, but seemed less suited for this application.)
Right above: The same LED inserted into the base of a reversed Zeiss 10X Kpl W eyepiece. By trial and error with optics to hand, this eyepiece focussed the beam to a ca 1 cm spot i.e. the approx. size of relay lens stop, thus improving light efficiency. The eyepiece focussed the LED's emission array in the rear focal plane of an objective in use, suggesting a well set up light system.
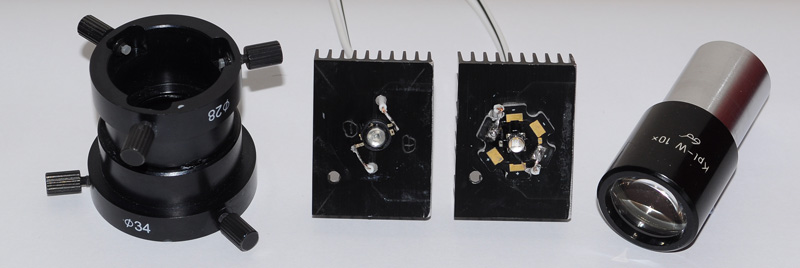
Two designs of power LED are shown attached to old CPU heatsinks (an LedEngin 400nm 1W and Luxeon Cyan 5W 495nm). The righthand LED design is a common one for power LEDs (the 455nm below is of same design) and fortuitously sits exactly within the base of the Zeiss 10X Kpl W eyepiece (the lenses are at top). This allows self centering and ensures LED is aligned with eyepiece optical axis. The adaptor left used to attach the eyepiece / LED unit to
the Zeiss epi side tube was made from two Moticam adaptors glued back to back.
After putting a dab of heatsink transfer paste at back of each LED, a tiny dab of superglue was put on each base periphery, then each LED attached to heatsink. This avoided use of screws so as to present an unobstructed surface to the eyepiece tube.
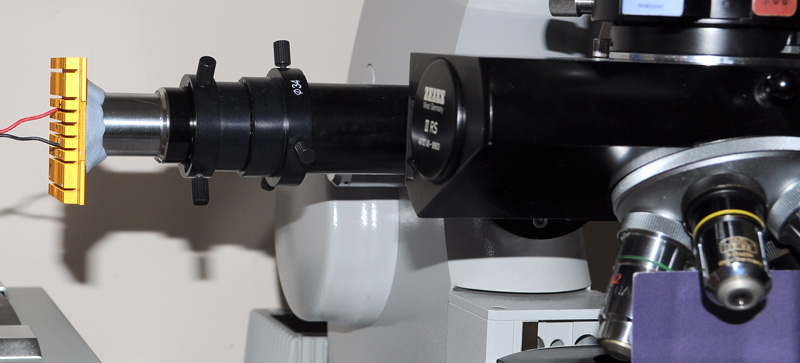
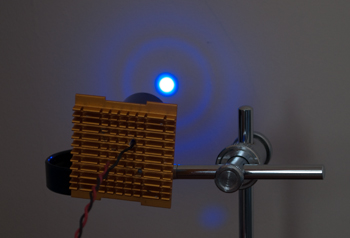
The epi-fluorescence setup showing an LED on CPU heatsink, inserted into base of the Zeiss eyepiece and in turn inserted into the centring adaptor with flange 'A' of epi tube removed.
For trials, the LED on heatsink is held in place in the eyepiece with a collar of BluTak as LED unit weighs very little and allows some fine centring adjustments (also stops any light leakage). A permanent design could use spring clips or a collar. The heatsink is luke warm after extended use at near full power.
The opaque card on stage is to block reflected light off slide from the observer. The power LEDs are intensely bright, deep blue can be dangerous to the eyes as well as near UV. When changing slides or objectives, the soft power button on PSU or the sliding light baffle on III RS head was used.
|
Right: The electrical setup for safe trials of high power LEDs with differing current requirements. The maker's data sheets describe recommended voltages / currents.
The compact switched mode power supply, although not bought for the purpose, is very useful for trials, as can be set to constant current or voltage. (Although this supply is overkill, a variety of cheap variable or fixed V/I PSU options are now available for LEDs.) The variable voltage allows the light intensity to be increased as well as monitoring the LED V/I so as to keep within its specifications. The 'soft' on/off for power
is also handy. Unlike mercury / xenon lamps, LEDs are tolerant of such switching (and exploited for multi-colour excitation for example in Zeiss' impressive Colibri LED fluorescence microscope system.)
A Philips Luxeon® III Star (5W, 1A, 3.9V, 450mW luminous flux) 455nm 'Royal Blue' LED is in use*, shown running at almost its max. current rating. Some of the 6.2V set is dropped across load resistors shown bottom left. These are 2 x 4.7 ohm 10W (70p each from Maplin) in parallel to give a safe load to the supply and LED. Although only ca. 5W
is being dissipated, the resistors still get quite warm and sit on a slate disc.
*Maker's code LXHL-LR3C, bought from Farnell UK for £5.36 + VAT (although min. total order £30 from this supplier).
|
|
In use for deep blue autofluorescence
Zeiss state in their IIIR RS manual that the default 'blue' excitation setup in the epi head is as follows:
Excitation: 2 x BG12 3mm filters - transmittance 0.04 at 510 nm and 0.01 or less at 530 nm. (Data from Optical-Filters.com for a 1 mm example.)
'FT 510' dichromatic mirror: - i.e. reflecting light to the subject <510nm and transmitting >510nm.
Barrier: 500nm, transmitting light above this wavelength'
LEDs have a narrow bandwidth, unlike the broad spectrum of light emitted by mercury or tungsten lamps; the Luxeon III data sheet for 5W 'Royal blue' states a max at 455 nm, half bandwidth ca. 20nm and emission at zero on a non-logarithmic spectrum by 500nm. This suggests that the BG12 excitation filters are not required. However, for the author's setup, this was certainly not true in practice, as the image sequence below shows.
(Images taken with a Nikon D5000 DSLR using a Zeiss 10X Kpl W eyepiece on short collar for projection.)
All images taken using a 'Royal Blue' 455nm LED at 1A with a Zeiss FT 510 dichromatic mirror. Barrier filter 500 nm.
|
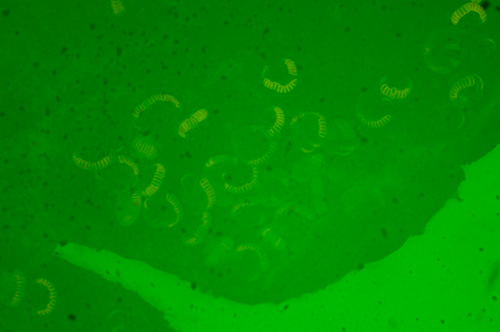
No excitation filter.
Silver fern leaf, Gymnogramma peruviana, dry Victorian mount.
Zeiss 2.5X planachromatic objective.
Exposure ISO 200, 2 secs.
A 'green wash' of light is dominant, masking any fluorescence. The dirt is under the coverslip, fluorescence imagery normally suppresses this as it doesn't fluoresce.
|
|
|
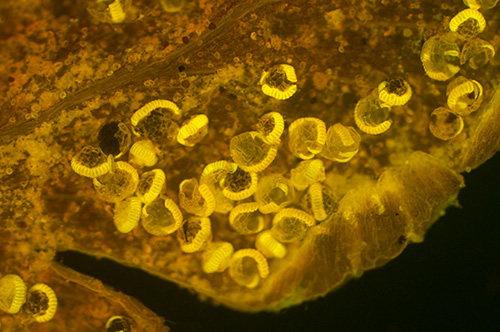
As above except 1 x BG12 reinstalled as a excitation filter.
Exposure ISO 800, 8 secs.
The green is completely suppressed giving a good contrasty fluorescence image and is very similar to that obtained with a 100W quartz halogen or mercury lamp with 2X BG12 filters.
The exposure is also identical to the 100W halogen, so no gain in visual or photo brightness for the LED.
|
|
|
1 x BG12 excitation filter in place.
Palate of whelk, Wheeler slide. Good contrast image and very similar to that seen with a 100W halogen or mercury lamp.
Leitz 6.3x NA0.2 NPL Fluotar.
Exposure ISO200, 2 seconds.
|

|
|
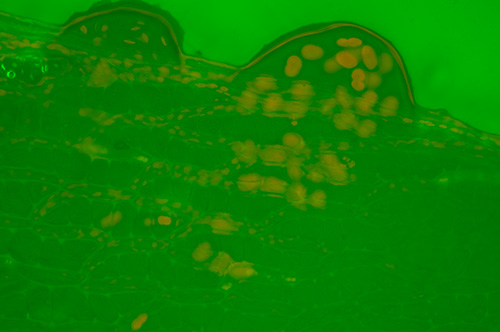
Fresh Sphagnum palustre leaf in aqueous mount with no excitation filter.
Again the green wash is seen, masking the fluorescence and suggesting the green is not dependent on certain slide types.
Zeiss 25X NA0.6 Neofluar.
Exposure ISO 200, 2 secs.
|
|
|
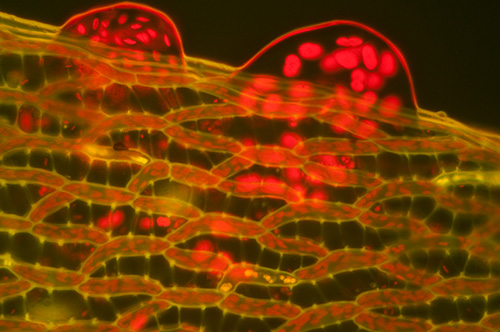
As above with 1 x BG12 excitation filter left in place.
Fresh Sphagnum palustre leaf showing the intense red autofluorescence from the chlorophyll containing structures and emission from cell wall structures.
Zeiss 25X NA0.6 Neofluar.
ISO 800, 10 secs. Out of camera image.
Again the penalty of using a BG12 filter is a much longer exposure and dull visual image.
|
|
Possible reasons for the 'green wash' when LED used with no excitation filter?
A number of possibilities spring to mind:
Small 'tail' of emission from 455nm LED above 500nm? Although the bandwidth at half intensity is narrow (20nm in this case) the LEDs do have a noticeable tail on the maker's spectra to just below 500nm. The maker's spectra suggest the tail extends to at least 30nm above and below the peak wavelength. Not having seen a logarithmic spectra, maybe the 455nm LED has a fraction of a percent transmission >500nm sufficient to swamp weak fluorescence. If so, should the dichromatic mirror and barrier filter cope with this? Increasing the barrier filter to a 530nm or even 550nm 'long pass' in absence of the BG12 still
showed a 'wash' of non-fluorescent colour to some extent.
A way to check this, is by inserting a narrow band ca. 455nm interference filter in the light train to cut off light above e.g. 490nm, but these filters are expensive in sizes large enough to cover the beam width. (See update below.)
Dichomatic mirror or barrier ageing? These are old mirrors / filters, but did replace with likely comparable modern equivalents off eBay and the exact same effect was seen. The spectra of the maker's dichromatic mirrors seem hard to source, but the Zeiss 'FT 510' is unlikely have a vertical cut off at this wavelength. Spectra seen for other comparable mirrors on eBay show a marked slope extending below and above the mirror's stated wavelength at half transmittance.
Autofluorescence of objectives, eyepiece relay lens or epi head optics? These are the only optics exposed to the intense blue, after that the optics train above mirror / filters is transmitting weak fluorescence emissions. Trials without eyepiece and epi side tube, ruled out these optics as autofluorescing. The LEDs are intensely bright and from previous studies Canada Balsam mounted slides do autofluoresce strongly in blue emitting a
similar
shade of green; if the objectives or other optics use balsam type cements, autofluorescence is possible. (Special fluorescence objectives are sold to minimise this effect.) However, a variety of objective types, Zeiss achromats and Neofluars, Leitz NPL Fluotar were tried and all showed it. Zeiss remark that Neofluars are particularly suitable for fluorescence studies.
Decreasing the light intensity did not seem to change the green wash intensity relative to the subject fluorescence.
Discussions with Laurent Delvoye suggest that his LED for fluorescence conversions did not show this green wash for blue excitation studies with high power LEDS, so maybe it is a quirk of the author's setup.
Comments to date
The author is uncertain as to what is responsible for the 'green wash' in the subject field for a narrow bandwidth LED 455nm source. The green can certainly be removed to give good contrast fluorescence imagery by retaining a 1 x BG12 excitation filter in place. But this filter only has ca 60% transmittance at 455nm so is partly acting as a neutral density filter; imaging with the 5W LED at full power with the BG12 filter is no brighter than using a standard 100W tungsten halogen
lamp. The author was hoping a 5W LED would give an intensity approaching that of a mercury lamp to improve visual studies. However, the LED blue fluorescence setup is more convenient in use than moving the 100W halogen lamphouse from transmitted to epi mode and the author is encouraged to try other LED wavelengths. The LED also gives very comparable results to a 100W halogen or mercury lamp, suggesting that the wide band blue excitation of these type of lamps is not vital for the good autofluorescence colour tones seen in the subjects studied to date.
Update March 19th 2011: 'Green wash' identified and cured.
A small 12mm diameter interference filter, 460nm with 20nm half bandwidth, was spotted on US eBay for less than £10. This was purchased and its size limitation overcome by mounting at the field stop of the eyepiece 'relay lens' in card collar (see below). Being close to the LED lens it fully covered the light beam. When this was retried with the 455 nm LED for blue excitation, without the BG12 excitation filter, the green wash had been eliminated and contrasty fluorescence images obtained, comparable to those using the BG12 filter.
|
Right: 455nm LED on heatsink.
Far right: Zeiss 10X Kpl W eyepiece with optics removed to show the 460nm interference filter on card base inserted at stop position. The eyepiece base has no optics in this design and sits snugly over LED base. If other LED wavelengths require an interference filter, the eyepiece field stop is a convenient place to insert other small, affordable filters.
|
|
The interference filter spectra was supplied and shows a sharper cut off as approach zero transmission than that of LEDs (see chart below). This suggests that on their own, one or both of the dichromatic mirror / barrier filter are ineffective at removing a small LED tail above 500nm. Laurent Delvoye may have been able to use the Leitz blue cube without excitation filter or interference filter because the Leitz mirror was to a tighter reflection / transmission specification.
|
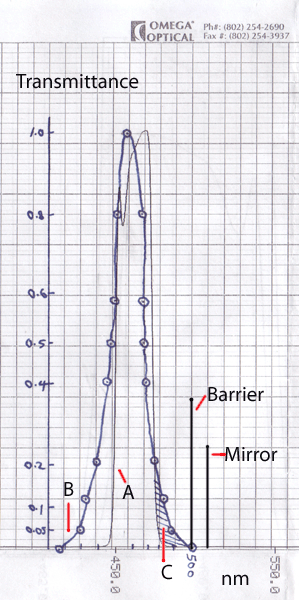
Chart right:
A - Interference filter 460nm half bandwidth 20nm ('460DF20', 12.5mm diameter) bought off US eBay, chart supplied with filter, courtesy of Omega Optical / eBay seller 'bjomejag'.
B - LED emission spectra of Luxeon III Star LXHL-LR3C, 'Royal Blue', 455nm half bandwidth 20nm. Approximate, redrawn from small spectrum on maker's data sheet.
The filter isn't a perfect match to the LED peak output so may cause some light loss.
The LED emission tail as approaches zero transmittance broadens and approaches the dichromatic mirror and barrier filter cut off. (The maker's spectra is not logarithmic, so uncertain if there's a small but finite emission above 500nm.)
C - Shaded area likely to be cut-off by using the interference filter in front of LED. The filter is shown to remove entirely the 'green wash' seen when no BG12 excitation filter was used.
Barrier - 500nm barrier filter in standard 'Zeiss blue' fluorescence excitation setup in III RS head.
Mirror - dichromatic mirror in 'Zeiss blue' setup. Note that dichromatic mirrors may not have sharp vertical cut-offs, 'Zeiss FT510' refers to the 510nm wavelength midway on the slope of reflectance / transmission transition.
|
|
From these limited trials, it suggests that an excitation filter, preferably an interference filter, may be needed with fluorescence systems retrofitted for LEDs, to prevent any emission tails from the LED passing the mirror and/or barrier and potentially masking fluorescence imagery.
Out of interest, the original patents of both the Olympus and Fraen epi and transmission LED fluorescence systems respectively were studied online, and both describe the use of excitation filters with the LEDs; Fraen mentioning that theirs was an interference filter.
The main aim of these trials was to see if a high power 5W LED gave a useful increase in brightness for blue autofluorescence than with a 100W quartz halogen. The exposures for the subjects tried are within 1 camera stop for both lighting systems, irregardless of whether BG12 filters or an interference filter was used, so there is no gain in this regard although the LED is more convenient for the author. The LED + interference filter combination may have the potential for giving more light if the filter is perfectly matched; there's a slight mismatch for the author's pairing which may decrease output.
The visual image was quite usable for brighter autofluorescing subjects, but not bright enough to use the Live View mode of a Nikon D5000 DSLR at lowest objective NAs.
The mercury 100W HBO lamp previously used, gave 3-4 more stops in this respect, which may be key for weak fluorescence, if the disadvantages can be tolerated in a domestic environment.
Comments to the
author David
Walker
are welcomed.
Footnote added July 22nd 2011. Since writing this article, I've read Ely Silk's valuable paper written in 2002 (3) where he discussed and illustrated the potential need for an interference filter with some LED fluorescence setups.
Acknowledgements: Thank you to my brother Ian, a retired electronics engineer, for loan of the power supply and advice on circuitry. Thank you to a fellow Zeiss enthusiast who generously donated the Zeiss epi side tube and spare excitation / blocking filters which came in useful. Thank you to Laurent Delvoye whose Micscape article on LED epi-fluorescence inspired my own adaptation for the Zeiss and who provided helpful discussions. Laurent notes that in turn he would like to credit Pieter Houpt whose own work inspired him and who helped Laurent to develop his own LED based systems.
Past 'Forays':
- Forays into fluorescence. Simple transmitted blue
light autofluorescence of mosses and algae imaged with a digital
SLR - trying
out recommended filters with darkfield to assess whether a consumer DLSR can
capture the weak imagery. Mar.09
- Forays into fluorescence 2: A selection of
prepared unstained slides studied in visible light fluorescence -
notes on and
images of a selection of slides that responded to this type of lighting.
May.09
- Forays into fluorescence 3: Exploring the 'BPAE' triply
stained microscope slide with or without fluorescence
equipment
- studies of
the complementary slide often sent to Nikon / Olympus image competition
entrants; includes a repeat of a study which illustrated that darkfield alone
with a halogen lamp can reveal the fluorescence imagery. Oct.09
- Forays into fluorescence - 4. Notes on the
autofluorescence of old mounts of snail radulae -
follows up an aspect of March article on exploring these
structures. Apr.10
- Notes on renovating a Zeiss III RS epi-fluorescence head and
adapting use of a spare port for axial epi-brightfield -
describes the exploratory repair of a 'cooked' example and finds it
a design suitable to work on. If only used with safe, suitable lamps, a
spare port can be adapted for axial epi brightfield studies. Includes small epi
photo gallery. Feb.10
Appendix: Comments on use of mercury arc lamps for fluorescence microscopy in a domestic environment.
Cost - each mercury arc bulb typically costs at least £90 in the UK and the 100W versions are typically rated for 300 hours. The 50W bulbs for the smaller lamphouses are often more expensive with shorter lives, ca. 200 hours.
Committed to long switch on times - the lives of these bulbs are reported to be significantly reduced if only used for short switch on periods; in the hobbyist setting this can be inconvenient as need to leave the bulb on for a safe minimum time which may be well beyond an intended working session.
Power supplies - the special expensive power supplies for the bulbs can be bulky and awkward to place in a space limited environment.
Safety - the mercury bulbs have potential safety issues:
At working temp. they are under high pressure*; although a small risk if they are used correctly, it is not unknown for the bulbs to explode. The lamphouse is designed to contain an explosion but can do a lot of damage to the lamp optics, as the photograph in Rost's book strikingly shows (2), the mirror and lamp optics were destroyed. (*Xenon lamps are always at high pressure, hot or cold.)
Although the bulb by inspection contains less mercury than a typical domestic thermometer, if the bulb explodes this mercury is in vapour form and potentially much more dangerous than the low vapour pressure of the cold liquid. A bulb exploding in a domestic environment can pose a dangerous safety hazard. Laboratories using mercury invariably have rigorous clean up and monitoring procedures after mercury
splls, (especially for the old high vacuum diffusion pumps that used liters of boiling mercury!). The high air throughput of a lab was also a safety feature, unlike the stale air of a typical domestic environment.
Intensity and UV: The lamps emit intense light in both the visible and near UV and need great care in use; a correctly setup mercury lamphouse and dedicated epi-fluorescence head with the correctly matched dichromatic mirror and excitation / barrier filters need to be used. Unlike LEDS, the intensity cannot be reduced electrically.
Reference.
1) 'Artifical lighting and the blue light hazard' by Dan Roberts.
2) F. W. D. Rost, 'Fluorescence microscopy', vol. I, CUP, 1992.
Ref. added July 22nd 2011. 3) Ely Silk, 'LED fluorescence microscopy in theory and practice', The Citizen Scientist*, July 19th, 2002.
*Magazine of the Society of Amateur Scientists, website www.sas.org (at the time of writing, the site's magazine archive doesn't seem to be working for 2002).
©
Microscopy UK or their contributors.
Revision: Published, March 13th 2011.
Updated March 19th 2011 with interference filter modification and examples.
Published
in the March 2011 edition of Micscape.
Please
report any Web problems or offer general comments to
the
Micscape
Editor
.
Micscape
is the on-line monthly magazine of the Microscopy UK web
site at
Microscopy-UK
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk
with full mirror
at
www.microscopy-uk.net
.