
Forays into fluorescence
Simple transmitted blue light autofluorescence of mosses and algae imaged with a digital SLR
by David Walker, UK
Professional fluorescence microscopy typically uses dedicated high intensity lamps with epi-equipment and more recently sophisticated laser techniques, but simple transmitted light fluorescence studies have been described in the literature for the enthusiast using a compound microscope and suitable quartz halogen lamp, filters and darkfield (ref. 1 - 3).
Another approach now possible for the enthusiast is the use of low cost, high power LEDs together with sensitive but affordable cameras. Ely Silk has provided an excellent comprehensive resource on his website www.viewsfromscience.com with detailed practical advice and striking examples of the results. Some microscope makers (e.g. Zeiss, ref. 8) also now offer LED based fluorescence models; third party kits e.g. by Fraen for transmitted light LED fluorescence are also available to retrofit to some modern microscopes.
I hadn't tried any sort of fluorescence studies before, so for my first foray I was interested in basic transmitted studies of selected plant autofluorescence using a microscope's 100W quartz halogen lamp for visible light excitation using darkfield with filters suggested in the literature; I was particularly keen to see whether my digital SLR was able to capture the weak images, given the high ISO and low noise performance of modern DSLRs. A longer term project is to see how the imagery from such a setup compares with those from a microscope with an epi-fluorescence head.
Basic plant chlorophyll autofluorescence can be studied using a deep blue excitation filter and an orange barrier filter (ref. 1). Leaves from mosses, liverworts and freshwater algae might be good practice subjects for such studies as they are easy to mount and give good imagery of the chlorophyll containing plant structures. A superb book which includes three chapters on the autofluorescence of plant, animal and other subjects is Volume II of Rost's 'Fluorescence Microscopy' (ref. 6).
Filter choice
Wratten gelatine filters
have been recommended and can be cut to suit, stacked if needed and can be protected from handling by mounting between glass (ref.
3). Small modern glass equivalents are now available e.g. from Astronomica,
often a comparable price and are more robust to heat and handling but there may
be fitting limitations of glass filters above the objective depending on microscope.
The author's Zeiss Photomicroscope accepts a 25 mm diameter filter in the main
accessory slot above the objectives. Lee Filters made of acetate could also
be tried, the filters' spectral characteristics are on the back of e.g
their 'ColourMAGIC' starter packs.
The Schott glass filters are an alternative and are available from optical and scientific suppliers, typically ca. £17 each in UK for 25 mm diameter, 3 mm thick (Knight Optical, UK). Although a higher initial outlay, the barrier / exciter filters in dedicated epi-fluorescence heads (e.g by Zeiss and Leitz) were often of the Schott type so the maker's fluorescence literature is useful for choosing filters for an excitation light of interest (or those suitable for a given fluorochrome dye) (ref. 4, 5).
I opted for two filter approaches summarised in table below; one set using filters to hand from the spares box but not necessarily well matched, the second a Zeiss matched set*. Only blue light excitation was studied.
*These
were salvaged from a
Zeiss III RS epi fluorescence head where all filters and dichromatic beam splitters had suffered
surface damage, possibly from heat, destroying the delicate beam splitters, but
the filters after home re-polishing looked excellent.
(Each
filter works on the epi head with its matched beam splitter which
reflects the excitation light onto the subject and only transmits the emitted light, but using darkfield for transmitted studies
helps prevent excitation light reaching the eyepiece.)
|
Filter and location |
'Blue Set 1': from the spares box |
'Zeiss blue' filters off epi III RS head* |
Others tried (as recommended in ref. 3) |
|
Excitation filter,
under substage; |
Schott
BG3, deep blue glass, (A BG3 is used in a Zeiss epi-fluor head with a deep violet UG51 for violet excitation). |
Deep
blue (Schott type) BG12 glass |
Wratten 47(B) gelatine, mounted on glass slide with large cover slip. |
|
Barrier filter, inserted above objectives, e.g. analyser slot if a feature on microscope |
Schott OG550, orange glass. |
'Barrier
filter 50' glass, |
Wratten 21 (glass) |
|
Other filters, red / near IR suppression**,**** |
CM500S
glass |
BG38 glass, 'red suppression'. |
|
* Zeiss III RS filter
data from ref. 4.
** A
red suppression filter is often a typical filter in epi-fluorescence
outfits because some excitation filters as well as transmitting in a chosen narrow band can also emit in the deep red / near IR,
e.g. the BG3 (ref. 4).
*** Filter thickness and /
or stacking same filter can be used to reduce residual unwanted light
in a filter's spectrum,
Zeiss use two BG12 filters in the III RS for blue excitation.
****
A heat blocking filter,
typically a KG1 is also a vital filter for typical epi-fluorescence with
mercury
arc and xenon lamps, possibly less important for transmitted darkfield
with a typical 100W quartz halogen
microscope lamp as none was fitted as standard on own scope.
Initial setting up trials
Excitation with transmitted brightfield
versus
transmitted darkfield
Darkfield is recommended for dyed filters to minimise
direct light reaching the eyepiece which may swamp weak fluorescence (ref. 2,3). But an example using blue excitation autofluorescence of algae is shown
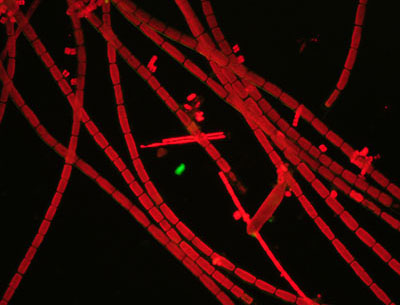
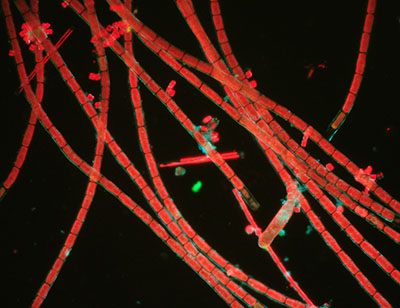
below in brightfield (left) and darkfield (middle, right) images.
Brightfield shows the residual blue light passing the exciter and barrier
filters but no observable fluorescence of the algae. Darkfield blocks direct
light and effective
at showing red emission from the chloroplasts and now a deep black background.
The third image shows the use of one BG12 filter in the Zeiss set rather than the stacked two for the middle image. The residual light passing the filters is significantly increased and passes a pale turquoise blue. The third image is thus a combination of darkfield in this light (of the cell walls) and the red fluorescence tonality modified by the blue. The combination lighting was useful for showing both non-fluorescing and fluorescing features for some subjects.



Subject:
Fresh filamentous freshwater algae, aqueous mount.
Objective: Zeiss 6.3X, NA0.16 planachro'.
Filters:
Zeiss blue, 100W halogen lamp, full intensity.
Condenser:
Zeiss brightfield 'J' setting, 'D' setting on phase, water immersion
Camera exposure: ISO 800, 20 secs.
Comments:
Images out of camera, apart from resize.
Just a darkfield
image or subject autofluorescence?
With darkfield confirmed as
the best lighting, a subject was tried that had little to no autofluorescence
but active in darkfield. From trials, processed cellulose fibres from toilet tissues showed
very little blue light fluorescence so used as a test subject. (Silica diatom
frustules seemed an option but found all my prepared slides had mountant autofluorescence).
Darkfield should be illuminating any refractive
part of the subject in the filter set leakage colour, whereas fluorescence will only show for plant structures
that fluoresce under the excitation light chosen, and in the colour expected
e.g. typically red for plant chloroplasts.
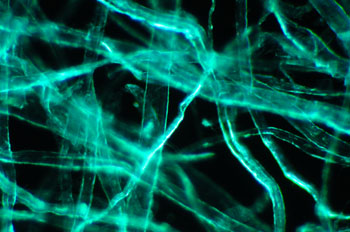
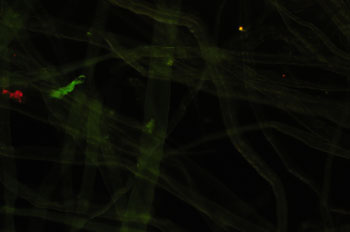
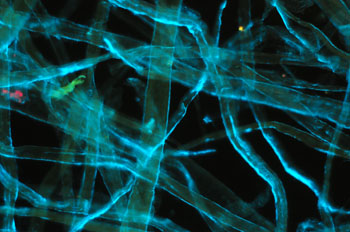
Processed cellulose
fibres as a weak to non-fluorescing control for blue excitation. Zeiss 16x/0.35
objective, 100W lamp full intensity except for lefthand image.
Left: normal
darkfield with a pale blue filter. (1/20th sec, ISO 800)
Middle: darkfield
with the Zeiss blue excitation filter set (2 x BG12, BG38, '50'), light leakage
minimal. (20 secs, ISO 800)
Right: darkfield with the Zeiss filter set but
just 1 x BG12. (20 secs, ISO 800). Filter leakage significant to show
a darkfield type image. But as remarked on below, this was found
useful to delineate the non-fluorescing features of a subject with fluorescence
included. (Mixed lighting is commonly used, e.g. epi-fluorescence with
transmitted phase and DIC.)
Visible studies
A
darkened room and dark adapted eyes are recommended for these sort of studies;
I did see a weak image by eye with binocular head under these conditions for the plants studied
and normal NA objectives, but personally found it impractical both from an inability
to really see detail and of microscopy in an almost dark room. This is where
a dedicated fluorescence microscope with high intensity mercury arc and high
NA objectives would be better. Although I'm fortunate to have access to
one, it does
not have a standard blue filter cube so direct comparisons weren't possible.
Digital imaging
I
used a Nikon D300 DLSR primarily, a current mid-priced model with CMOS APS-C sensor
but see quick comparison with a Nikon D50 DSLR below. It
has ISO 200 - 3200 as standard settings and shutter speeds to 30 secs plus bulb;
also a Live View feature using the main sensor. It was used exclusively
with Nikon's Control Pro 2 software to remotely control and instantly transfer
and assess the results on a PC.
Focussing - despite weakly visible fluorescent
images, there's an easy workaround to focus. Just prior to a fluorescence exposure,
the filters were replaced with a single red filter on the field lens to
give standard darkfield imaging, plenty bright enough to focus using Live view
(or camera viewfinder or parallax eyepiece if preferred). As the colour
was in a similar band as that emitted under fluorescence the focus plane
should be close to if not exactly correct.
Exposure measurement was
by trial and error, but as for normal photography it's surprising how quickly
the correct exposure can be judged to within a stop or so. On the author's scope
/ camera combination, ISO 800 and exposures from 10 - 20 secs coped with most
of the subjects tried with 12V / 100W lamp always set at full rated voltage and
was a good compromise between greater noise on higher ISO and excessively
long exposures. I didn't use the camera's built-in noise reduction software.
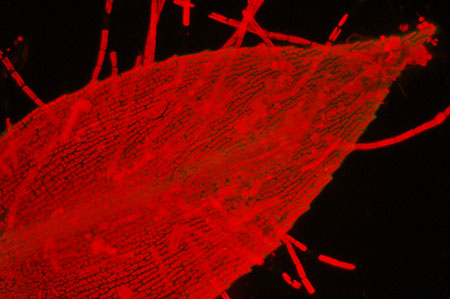
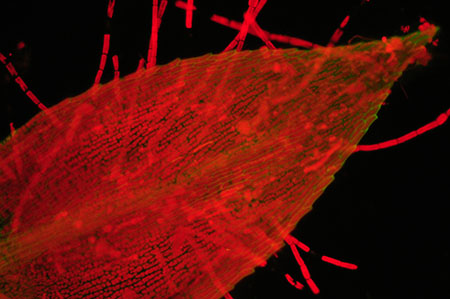
Moss leaf with epiphytic
algae. Left: Nikon D50, Nikon's entry level model 2005, right Nikon D300, current mid-range model.
Out
of camera, resized. 15 secs, ISO 800. Zeiss 16x/0.35 objective. Zeiss blue excitation
set. Oiled 'D' darkfield.
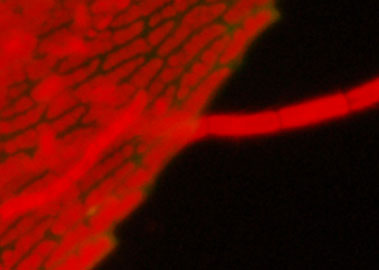
Detail from above.
Crops and resized to be comparable. Left Nikon D50 (6 Mpixel, CCD sensor), right
Nikon D300 (12 Mpixel CMOS sensor).
An older budget DSLR like the D50 seems competent from a quick comparison, although the D300 seemed to pick up the weak green for this example. 'Pixel peeping' showed more colour noise if lightened the black at 100% images cf the D300, but unlikely to see in web images and typical print sizes.
Some Results
Lamp:
Zeiss 12V, 100W transmitted microscope lamp at full intensity. All light sent
to camera on Photomicroscope beam splitter. Optovar 1.25X.
Camera: Nikon
D300 DSLR. White balance 'Auto'. Images all taken as jpegs (i.e. 3x8 bit RGB,
the RAW image offers 14 bits
a channel). 'Vivid' mode set to combat the camera's tendency to flatten colours
on a microscope when jpeg set to 'neutral'.
Condenser: Darkfield 'D setting on phase condenser, water or
oil immersed. There didn't seem much observable advantage in using oil on the
Zeiss phase condenser, so used water in the main. A trial of the Zeiss high NA ultra
darkfield oil immersion condenser gave
about a stop better exposure but no marked difference otherwise, so phase condenser
with darkfield setting was found more
practical.
Filter sets:
Zeiss
blue: Excitation - 2xBG12 (each 3mm thick) + BG38 red suppression. Barrier
- 'filter 50' pale yellow.
Blue set 1: Excitation - BG3 (3 mm thick)
+ CM500S red suppression. Barrier - OG550.
Subject:
Two fresh sphagnum leaves (S. palustre?), base and tip, water
mount. The deep black background and high contrast suggests the filter combination with water immersed 'D' setting of condenser is an effective one. The image right is a comparable view of same subject in brightfield. The blue-green chlorophyll of the algae in the hyaline cells can be compared with the paler green of sphagnum leaf chloroplasts. |
|
Subject:
Dead sphagnum leaf (S. palustre?), water mount. In addition to fresh green sphagnum in the sample taken from a local wet green lane, colourless branches that had died were seen. Fluorescence shows that a dead leaf is devoid of red emitting from chlorophyll but more clearly shows the green emission from cell walls. To confirm this was fluorescence rather than an artefact of weak transmitted light from the filters, a similar green image was obtained on a Leitz scope with epi-fluorescence head and blue excitation light. Although not sure what compound this is attributable to, cutin perhaps, comments welcomed. (Cutin in mosses has a 'whitish' emission in UV, ref. 9.) The small red area areas are chlorophyll containing algae attached to leaf.
|
|
Subject:
Fresh sphagnum leaf (S. palustre?), detail of leaf. Fluorescence: A comparison of how autofluorescence can complement other techniques for plant studies such as phase, shown in first image. Using the one BG12 filter mixes darkfield and chloroplast fluorescence to help provide detail in the cell walls. |
|
Subject:
Fresh sphagnum leaf (S. palustre?), leaf tip. The sphagnum leaf has a naturally rolled tip which is evident from this unflattened example. The water film held by the leaf is often host to a variety of microscopic organisms like rotifers. Using the one BG12 filter mixes darkfield and chloroplast fluorescence to help provide detail in the cell walls adding to the weak green fluorescence. |
|
Subject:
Freshwater filamentous algae. The fluorescence image can be compared with the brightfield phase image of same subject below it. Only the chlorophyl containing chloroplasts emit. The homebrew 'blue set 1' filters set were used, not the exact 'Zeiss blue' pairing. This and other studies suggests the set works well for red emission but as the orange barrier sharply blocks light below 550 nm it doesn't pickup any weak green emission. |
|
Subject:
Live, immobile volvox The sample of filamentous algae collected from a local pond also contained live volvox, and was curious to see if the algal colony would fluoresce in blue light. The specimen obligingly was rock steady under the microscope allowing the long exposure. The brighter areas are the daughter colonies. The same specimen (16x objective) in brightfield with DIC and lambda wave plate is shown right. The optical sectioning of DIC shows the surface algae clearly, I think the red background to the above image is the out of focus algae contributing to the image. |
|
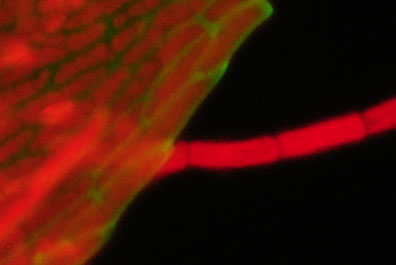
Subject:
Leaf from submerged freshwater moss from garden pond. This leaf has the more typical structure of a moss cf the distinctive form of the sphagnum above. The red chloroplasts are clear in both the leaf and epiphytic algae. Only one of the BG12 filters was used here which from earlier was shown to leak a pale blue darkfield image. This emphasised the cell wall structure and changed the red tonality. |
|
Comments to date
From
trials to date the images looked
quite usable from modest NA objectives, giving genuine fluorescence
and providing greater insight into plant structures. The backgrounds were a good black with little to no
post-capture image processing, suggesting the 'D' setting of the Zeiss condenser
was working well with the filter pairs used. The in-optic darkfield setting
of this type of condenser could well be superior to a simple patch stop in filter
tray. Both a modern and earlier model DSLR tried seem very capable of imaging
at the mid range ISO settings and long time exposures required.
Apparently plant tissue autofluorescence is typically much brighter than for animal tissues (ref. 6a), so the subjects chosen above may have been within the capabilities of the transmitted set-up with a DSLR.
Simple blue light fluorescence studies following procedures recommended in the literature has certainly opened up to the author a new way of studying suitable plant subjects with imagery totally different to that by brightfield and normal contrast enhancement. For readers with a DSLR and microscope with darkfield and halogen lamp, the outlay of suitable filters is worth considering.
Comments to the author David Walker are welcomed.
References
and Resources
1) W. Nachtigall, 'Exploring with the microscope', Sterling
Publishing, 1995, p. 40.
2) W. G. Hartley, 'Fluorescence in amateur microscopy',
Quekett Journal of Microscopy, 1978, 33, 372 - 375.
3) D. J. Cook,
'Fluorescence microscopy using gelatine filters and quartz-iodine illumination',
Quekett Journal of Microscopy, 1980, 34, 113 - 118. This author used
a Leitz 35 mm slide projector as light source.
4) Zeiss West, 'Epi-fluorescence condenser III RS. Operating instructions.', 12
- 13. Downloadable from Zeiss website and www.science-info.net.
5)
Leitz Wetzlar, K. Koch, 'Fluorescence microscopy. Instrument, methods, applications', 1972, 10 - 16. Downloadable from www.science-info.net.
Includes an excellent practical introduction to fluorescence microscopy.
6)
F. W. D. Rost, 'Fluorescence microscopy', vols. I and II, CUP, 1992. Obtainable
by interlibrary loan or a local university library may have it
and can also be 'dipped into' on Google Books: 'Vol.
II, Ch. 10, 'Autofluorescence in plants, fungi and bacteria'. Vol. I includes
a chapter 'Commercial fluorescence microscopes' covering both older and more
recent designs, some of which may be in the amateur's budget on the used market.
6a) Above ref., Vol.
II, page 16.
7)
B. Herman, 'Fluorescence microscopy', Bios Scientific Publishers, RMS Handbook
No. 40, 2nd edition 1998, p. 19.
8) B. Hohman, Carl Zeiss, 'LED Light Sources: A Major Advance in Fluorescence Microscopy', Microscopy and Analysis, November 2008.
9) G. Nordhorn-Richter, 'Primary fluorescence of mosses', Leitz Scientific and Technical Information, 1984, 8, 167 -170. A useful ref. from Rost's book, ref. 6.
Published in the March 2009 edition of Micscape.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK web site at Microscopy-UK
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk
with full mirror
at
www.microscopy-uk.net
.