Forays into Fluorescence - 4
Notes on the autofluorescence of old dry mounts of snail radulae
by David Walker, UK
In last month's article on studying the radulae ('palates') of snails using various lighting techniques, only a single dry mount responded particularly well to epi-fluorescence and autofluoresced brightly in visible light. Of the techniques tried it seemed one of the best to show the often complex three dimensional structure of this organ but the balsam mounted slides precluded using this technique because of the mount's own strong fluorescence. I remarked that it would be interesting to learn of other studies of dry mounted examples to see if this autofluorescence was more widespread.
A British reader, Brian, very generously offered four old dry mounted radula slides from his collection so has offered the opportunity to further explore this feature. The four slides are shown below, together with one slide from my own collection that has a similar mounting style.


Left: the four dry mounted slides kindly supplied. Labelled '8.51' and their style suggests they are early slides dated 1851.
Far right: a slide from the author's collection mounted in a similar style. (The 'Fisher' label is likely to be the much later owner not mounter, see last month's article.) Initially thought to be a balsam mount, from comparisons with the new four slides, it is more likely a dry mount.
Right: a 'J&TJ' slide for incident lighting that responded very well to visible epi-fluorescence in last month's article.
Notes on images:
Camera - Nikon D5000 DSLR on Zeiss Photomicroscope III using Zeiss 10X Kpl W eyepiece on short collar for projection.
Auto white balance, colours out of camera, resized no sharpening. Some tonal level adjustments where necessary if images tonally flat or to correct exposure.
Fluorescence using Zeiss III RS epi head with a Zeiss 100W quartz halogen lamp at full power.
Blue excitation: Zeiss set: 'FT 510' mirror or '520LP', Schott BG12 excitation filter, barrier '50' filter. The 'FT510' is the ideal Zeiss mirror which have sourced after the III RS head renovation.
Green excitation: Zeiss set: 'FT 580' mirror, 'BP546/7' interference filters, barrier filter 'LP590'.
'HFW' - horizontal field width in mm.
'Zonites cellarius'

I've found old references to this species including British records, but no modern refs. to date, so unsure of the mollusc's current status. The Wikipedia entry for the genus describes them as 'mostly small, air breathing land snails'. I'm uncertain if the dull brown / orange of the substrate is fluorescence or a weak brightfield epi image because of residual pass through of the filter / mirror set, but the yellow / green fluorescence for the 'teeth' is similar to that seen for the 'Palate of whelk' shown in last month's article.
Zeiss 10x NA0.3 planapo objective, blue excitation, ISO 800, 2.5 sec exposure. The image is readily seen by eye in a slightly darkened room using a normal quartz halogen lamp as the epi source. HFW = ca. 0.8 mm.
'Neritina fluviatilis'

This is a well recorded species in freshwater and apparently now Theodoxus fluviatilis. Fitter and Manuel (1) described the species as 'A common but inconspicuous species found on stones and other firm substrata.' It's stated to be 'up to 12 mm wide'.
The weak brown/ orange emission I was uncertain was fluorescence on the previous species is dominant here, with attractive hue differences to emphasise the different structures, so suggests this is a fluorescing material. Perhaps a hint of the green-yellow fluorescence is seen in the second row of off-centre 'teeth'.
The above slide is the donated slide, the author's own slide of this species gave very similar imagery after restudying it with blue excitation.
Leitz 6.3x NA0.2* NPL Fluotar objective, blue excitation, ISO 800, 10 sec exposure. HFW = ca. 1.3 mm. (*The slightly higher NA of this objective cf the Zeiss 6.3x NA0.16 planachro is useful to give brighter images, as epi brightness is proportional to the fourth power of the NA.)


Neritina fluviatilis with the same objective and field of view as for the fluorescence study, but using transmitted brightfield (left) and darkfield (right). The highly refractive structures in a dry mount were difficult to image successfully and reveal structural detail. For reasons I'm unclear of, the radula when self luminous in fluorescence also seemed to have a better depth of field.
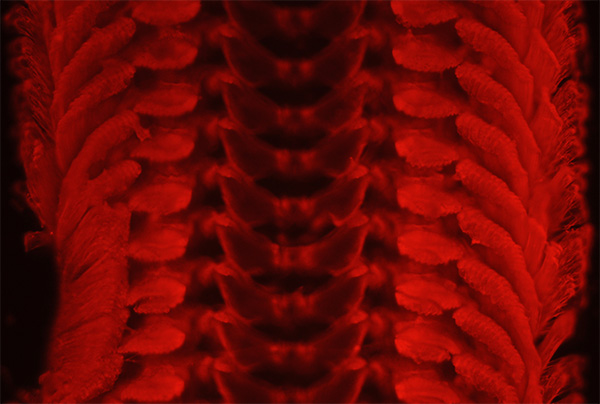
Neritina fluviatilis. All the radulae studied emitted in the red with green excitation to a greater or lesser extent. There was very little difference in the hue or tonality unlike with blue excitation so didn't find it as useful for studying the structures although it can give quite attractive imagery. Zeiss 10x NA0.3 planapo objective, green excitation, ISO 400, 10 sec exposure. HFW = ca. 0.8 mm.
'Grey slug Limax maximus'
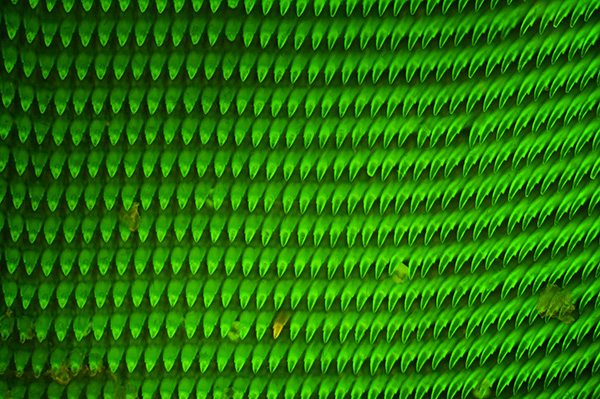
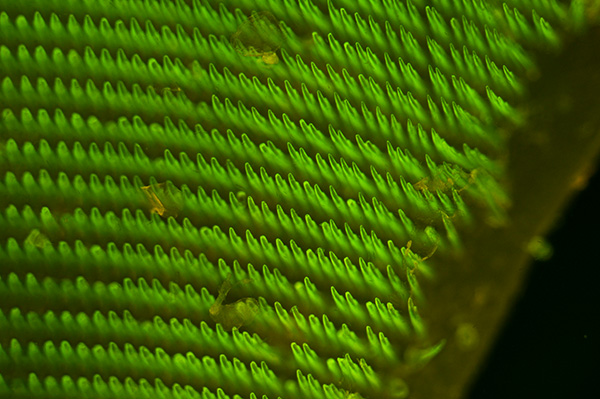
The great grey slug is one of the largest of the keeled slugs found in a typical garden (2). The underlying substrate did weakly emit in the brown / orange but because of the 'teeth' density, it was more obvious at the edge of the radula, as shown in the second image. Zeiss 10x NA0.3 planapo objective, blue excitation, ISO 400, 2.5 sec exposure. HFW = ca. 0.8 mm.
The two images above show the slightly different tonality from the author's original blue epi set with 520 LP mirror (upper image) and the now correct Zeiss LP 510 mirror (lower image), recently sourced after a long search. Although exact tonality also critically depends on the exposure, a deeper green is seen if err towards underexposure.
'Helix aspersa'
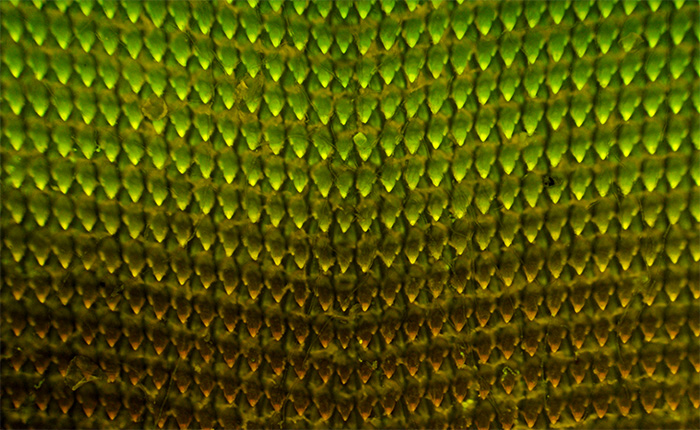
Helix aspersa is regarded to be the common 'garden snail' as noted in the Wikipedia entry for this species. Some of the radula mounts, as shown above, in transmitted visible had areas of the radula appearing cleaner of other material and it was these cleaner looking areas that showed the strong yellow-green emission (upper half of image), with less clean looking areas being dominated by the orange / brown emission. I'm uncertain if this reflects a variability across the radula in the effectiveness of treatment protocols during slide preparation. Leitz 6.3x NA0.2 NPL Fluotar objective, blue excitation, ISO 400, 5 sec exposure. HFW = ca. 1.3 mm.
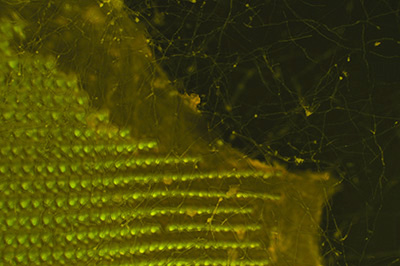
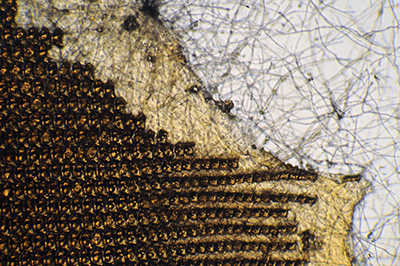
Helix aspersa. Another benefit of fluorescence is the potential suppression of either none fluorescing material such as detritus or material more weakly fluorescing than the subject such as an extensive fungal growth on the whole subject, as shown here. In transmitted brightfield, especially with contrast enhancing techniques, the imagery of debris or other structures may be distracting.
Comments to date: Radulae are typically described as 'horny' and/or 'chitinous', two materials that are reported to autofluoresce. Whether the type of processing of a radula plays a role is unclear, as some processes e.g. using formaldehyde are known to create fluorescing compounds with some subjects ('induced fluorescence', ref. 3). From the limited literature I have access to on autofluorescence, it seems that the exact nature of autofluorescence in some subjects isn't well known (3a), possibly because of the difficulties in isolating and identifying likely molecules in complex organisms.
Whatever the nature of the fluorescence, from the very limited number of slides tried, it does seem a useful technique for studying certain radula structures with the light microscope; transmitted techniques often give hard to analyse imagery. I would be interested to learn if the method is used by professionals as haven't sourced any information to date on radula autofluorescence. Perhaps the professionals' ready access to SEM imagery is more suited to three dimensional studies of such structures.
But for the enthusiast the appeal of fluorescence to the author at least, is that where a radula responds to such lighting, a clear indication of the three dimensional structure is shown. Blue excitation was the most useful for the radulae tried. In transmitted visible light the images can be complex and hard to interpret, especially with dry mounts as they are more refractive if mounted dry. Fluorescence also suppresses any detritus or other material such as fungal growth if these structures do not fluoresce.
Comments to the author David Walker are welcomed.
Acknowledgements:
Many thanks to Brian, a British microscopy enthusiast, who kindly and generously donated the four dry mounted slides described and which have provided many hours of rewarding viewing and photography. Also for his useful comments on these slides and on aspects of my own slides.
References
1) R. Fitter and R. Manuel, 'Collins Field Guide to Freshwater Life', Collins, 1986.
2) M. Chinery, 'The Natural History of the Garden', Fontana / Collins, 1978, p.80.
3) F. W. D. Rost, 'Fluorescence microscopy', vols. I and II, CUP, 1992, Chapter 12.
3a) As above, Chapters 9 and 10.