Forays into Fluorescence - 3
Exploring the 'BPAE' triply stained microscope slide with or without fluorescence equipment
(Complementary slide to Nikon Small World / Olympus Bioscapes competition entrants)
by David Walker, UK
Updated November 2009 prompted by a reader's phase, oblique results
Participants in the Nikon Small World and/or Olympus Bioscapes competitions in recent years were often treated to a complementary prepared microscope slide. A common slide sent is the so-called BPAE slide 'Bovine Pulmonary Artery Endothelial Cells' triply stained for fluorescence studies.
I received my first example from one of last year's competitions but at the time didn't own any fluorescence equipment so I filed it away as potentially useful in future. As others have remarked, it seemed to reveal very little by brightfield or phase (see update below), but as Walter Piorkowski reported and strikingly illustrated in the Amateurmicrography.net Forum and a later thread, the slide can yield impressive results with careful use of a darkfield condenser with an unfiltered halogen lamp, providing a flavour of the multispectral cellular imagery seen with a fluorescence microscope.
Summarised below are examples of the way this slide can be useful based on the author's own studies and following up the darkfield lead remarked on. This is from the perspective of the author on a steep learning curve in enthusiast level fluorescence microscopy and with no expertise in the biology underlying BPAE type slides. (As an aside, if recipients have no interest in the slide, they may wish to consider donating them to fellow enthusiasts or colleges that can make use of them; in the UK a comparable BPAE slide is expensive to purchase.)

Two recent examples of the complementary BPAE slide
The BPAE slide - A Molecular Expressions article summarises the history of the 'BPAE line' its importance in biology and the association of the three fluorochromes with different cellular structures, with links to images. A striking Nikon MicroscopyU video clip of the live cells also labels the cell structures.
'Molecular Expressions BPAE Bovine Pulmonary Artery Endothelial Cells' in Cytoseal - fluorochromes stated on label.
|
Fluorochrome |
MitoTracker Red CMXRos |
Alexa Fluor 488 - Phalloidin |
DAPI |
|
Excitation wavelength, peak max |
Yellow Green 579 |
Blue 495 |
Near UV 350 |
|
Emission wavelength, peak max |
Red 599 |
Green 519 |
Blue 470 |
|
Cell component shown (ref. 2) |
the network of mitochondria within the cell |
the network of actin (a protein) filaments |
DNA in nucleus |
Sources: 1) Olympus Fluorochrome Data Table. 2) Molecular Expressions Fluorescence Digital Image Gallery
Potential uses of the BPAE slide:
Brightfield
- as a reference for studies below
Darkfield
- a demanding but very rewarding test of a well setup NA darkfield condenser
- if successful, an opportunity to study fluorescent techniques and how different fluorochromes are used for showing cell structures
- the use of a high sensitivity camera, eg digital SLR or microscope camera to capture the imagery
- exploring how inserting different filters into the light train affects the imagery
Fluorescence
- extremely useful reference slide if setting up fluorescence because the triply stained BPAE is very well documented
- comparing the image intensity of the fluorochromes using different light sources such as a 100W tungsten halogen and a typical mercury arc lamp
- exploring the performance of different excitation / blocking filters to learn how these must be matched to each fluorochrome's absorption / emission properties
- exploring the cell structures revealed at different excitation wavelengths
- optimising image capture with camera available, exploring e.g. the noise and other camera parameters for a fluorescence subject
- trying image merge techniques to create a single multicoloured image from separate excitation studies, either in camera or in software
Brightfield with contrast enhancement
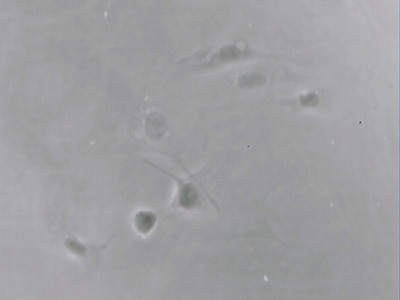
Human cheek epithelial cells in aqueous mounts are a classic demonstration of the value of contrast enhancement techniques. But, suspect like others, I found the BPAE endothelial cells only barely visible with phase, oblique, COL and even DIC. A typical image with phase is shown later next to the same field of view under fluorescence, parts of some cells were only just seen despite the cells being present in high density.
In the Nikon MicroscopyU video clip of live BPAE cells in DIC, i.e. in aqueous media the cells are clearly visible. The very low contrast on the prepared slide may suggest the refractive index of the Cytoseal mount is very close to the cellular structures after the preparation and/or very little absorptive/refractive features. The RI for Cytoseal is 1.48 at 23 șC i.e. almost the same as glass RI 1.50 (live cells would be in water based medium with an RI of ca. 1.0). I'd be interested to hear from readers familiar with this mount as to visibility or lack of in brightfield with different mountants.
Update Nov. 2009: Thank you to Mike Gibson who sent me his own very clear, contrasty images of the BPAE slide in both oblique and phase using a LOMO Biolam system. Prompted by Mike's results I tried my own LOMO instead of the Zeiss system used in the article but wasn't able to obtain comparable imagery with oblique or phase; my own LOMO phase images were slightly better than with the Zeiss but still weak, no oblique imagery was detected. Perhaps there's some slide variability that affects the ease of such imaging, comments welcomed.
|
|
Image and details courtesy of Mike Gibson. LOMO phase 10x objective. |
|
LOMO oblique was tried by author but no detectable imagery at all. |
|
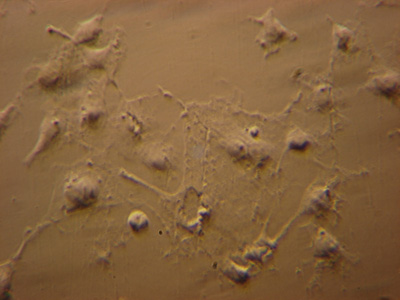
Darkfield - low intensity lamp, LOMO Biolam with darkfield condenser
Obtaining good results both visual and photographic is probably more of a challenge with the typical low intensity 6V 15W external lamp supplied with the LOMO and many other microscopes.
Tips to maximise image intensity and contrast:
- if scope has a binocular head, replace with a monocular head and low power eyepiece if available
- use a high NA objective
- as there is little to focus on the BPAE slide, optimise the darkfield first with eg a diatom strew, then leave condenser focus and darkfield centering alone and replace with the BPAE slide
- the visual image may be weak so try a darkened room to dark adapt the eyes
- glare from the slightest dirt or smallest bubble in oil can affect the image at full lamp intensity, rigorously clean the slide and check oil for bubbles
- carefully adjust condenser darkfield focus and field iris to minimise glare even if only the central small area has true darkfield
With the author's LOMO Biolam setup described in the image caption below, there was a bright enough visual image with dark adapted eyes. The image was just bright enough for a consumer digicam to focus on the image. It's instructive to try different objectives, as the textbooks note* that the brightness of transmitted fluorescence is to the square of the objective NA and inverse of the mag, so a compromise has to be found.
* e.g. B. Herman, 'Fluorescence microscopy', Bios Scientific Publishers, RMS Handbook No. 40, 2nd edition 1998, p. 19.
|
Image right: LOMO Biolam, LOMO OI-13 darkfield condenser oiled to slide. 6V 15W OI-19 tungsten lamp, max intensity, no filters. Unnamed 40X NA0.74 objective. Monocular head. Sony P200 consumer digicam on 3x optical zoom supported on LOMO 7x eyepiece. Exposure 8 secs, ISO100, f5.2. Colour and tonal balance out of camera, resized and some resharpening. A tungsten lamp of this wattage has very little near UV component to excite the DAPI, so the nucleii stained with DAPI only show a feint hint of the blue emission light. Compare with images from quartz halogen and 100W mercury lamp below. Any particles in the mountant, on the coverslip or air bubbles in oil will affect image as shown by the bright specks. |
|
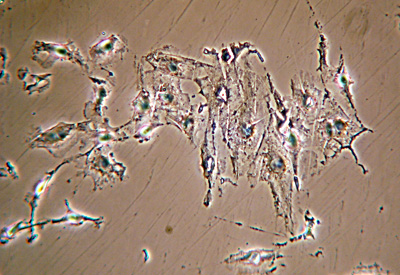
Darkfield - high intensity lamp 12V / 100W quartz halogen lamp, Zeiss photomicroscope and Zeiss ultra darkfield NA1.2 - 1.4 condenser
The approaches to optimise the results were similar to that for the Biolam above, but the much higher intensity lamp gave brighter visual images suitable for binocular head studies in normal room lighting. A 100W halogen lamp also has a small but usable near UV component, so the images showed a greater emission of the nucleii in blue from the DAPI stain. Compare the image below with that from the LOMO above.
Trials with the immersed darkfield 'D' setting on the Zeiss phase condenser were unsuccessful, the darkfield didn't seem to be of a high enough quality with a typical 40X NA0.75 objective to avoid glare swamping the weaker fluorescence.
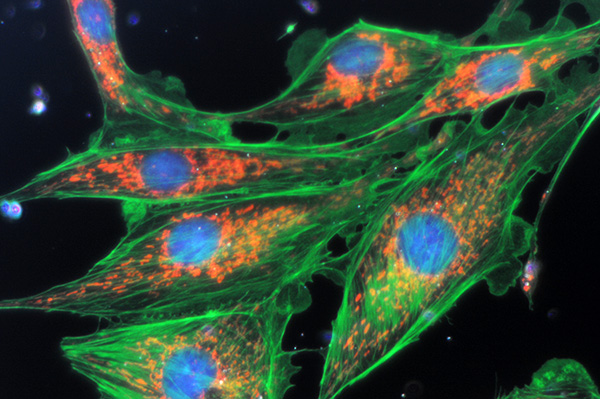
Zeiss 40x NA0.75 objective with 100W tungsten halogen lamp at full intensity and oiled Zeiss ultra darkfield condenser. No filters. Exposure 8 secs ISO400. Nikon D300 DSLR. Interestingly, the exposure required was longer than the LOMO despite the more powerful lamp. Possibly because of the 'lossier' optics, projection method and critical settings of darkfield condenser. Some tonal balance adjustment, resized without sharpening.
There's sufficient near UV from the lamp to excite the blue emission from the nuclear staining.
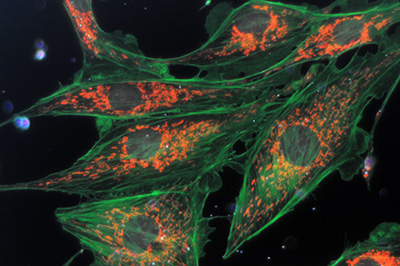
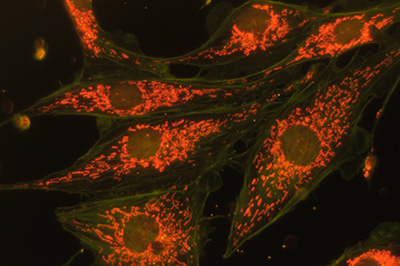
Same image field as above. With this full spectrum excitation with white light, the effect of different filters in the light train can be tried.
Left - a 420 nm long pass filter on field iris blocks the near UV from the lamp thus losing the blue DAPI emission of the nucleii.
Right - an orange filter above subject blocks most green and allows mainly the deep red emission to be seen.
Why is the fluorescence of the BPAE cells visible in darkfield with unfiltered tungsten lighting?
I would be interested in readers thoughts on the accuracy of the following interpretation. Transmitted fluorescence microscopy with darkfield is well established but usually requires suitable filters below and above the subject slide with a microscope lamp to prevent the directly transmitted light giving a typical darkfield image (see Forays into Fluorescence - 1 for examples).
If the cells had any significant absorption or refractive structures in brightfield, the cells would probably have been readily visible with the high intensity darkfield in the full spectrum white light, thus swamping any weak fluorescence emission. But because the cells are near transparent, they show little to no darkfield imagery so excitation and blocking filters aren't required. But the cells are being bathed with intense white light, so the weaker fluorescence emission can be seen. The blue, green and finite component near UV from a quartz halogen lamp excites all three dyes together (or two dyes with the weaker low wattage tungsten lamp).
Fluorescence studies
A Leitz Diaplan with Ploemopak head was used for traditional epi-fluorescence studies. Ironically the visual image and photography was in one respect less satisfying because all three dyes are not being excited together to see a tri-colour emission. Each dye is now excited with an appropriate filter cube, with filters for excitation and blocking with dichromatic mirror. What is immediately apparent is that the field is no longer affected by detritus or slide imperfections causing glare from darkfield, as only light from fluorescence is now being studied.
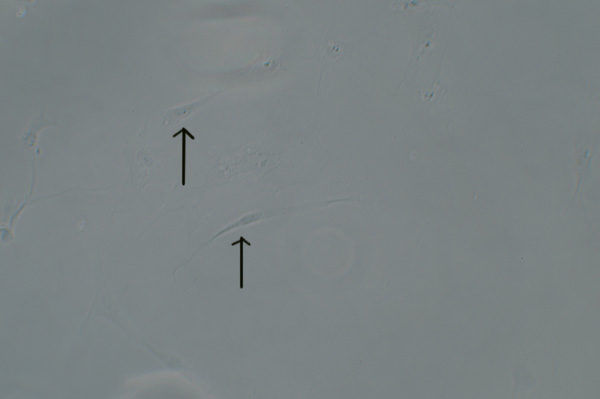
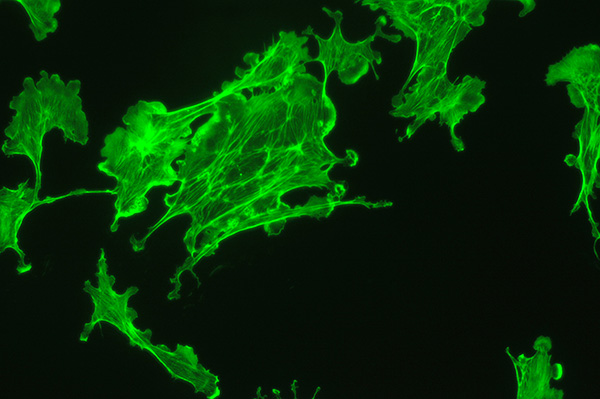
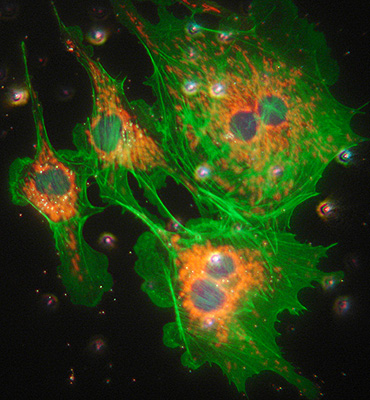
Above: Transmitted phase compared with fluorescence for same field of view. Zeiss 16x NA0.40 objective.
Upper image: brightfield phase with 100W halogen lamp only shows a hint of parts of the BPAE cells. DIC or oblique is no better.
Lower image: same field with epi blue excitation from 100W mercury lamp. Exposure 4 secs ISO400.
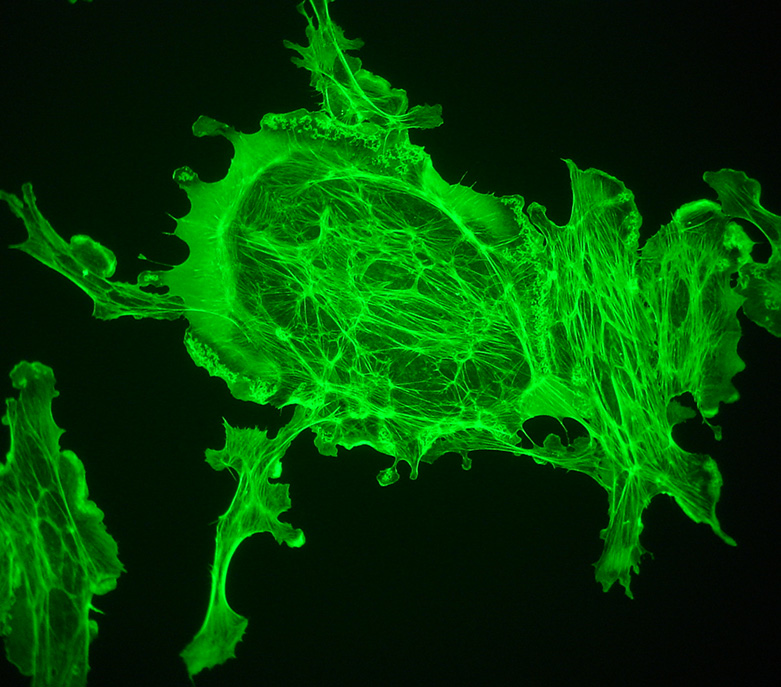
With the fluorescence microscope and 100W mercury lamp, the images are bright enough for a consumer digicam such as the Sony P200 to be supported over an eyepiece and allow it to auto expose and focus. Exposure 2 secs ISO100. The actin filaments are particularly well shown in this example. 40X objective.
Image merging trials
Professional microscopists carrying out fluorescence studies will often merge images from a stained subject at different excitation wavelengths to create a composite image. The primary images may be monochrome using R, G, B filters and coloured correctly in software as shown in this Molecular Expressions example of the BPAE slide.
The enthusiast can try similar merging techniques. Another approach is to use the 'Multiple Exposure' function offered by some consumer digital cameras; three coloured images can be taken and then combined in the camera. The Nikon D300 permits up to 10 images to be merged. The camera manual notes that it uses RAW images to do this so can be superior to other post capture merging methods.
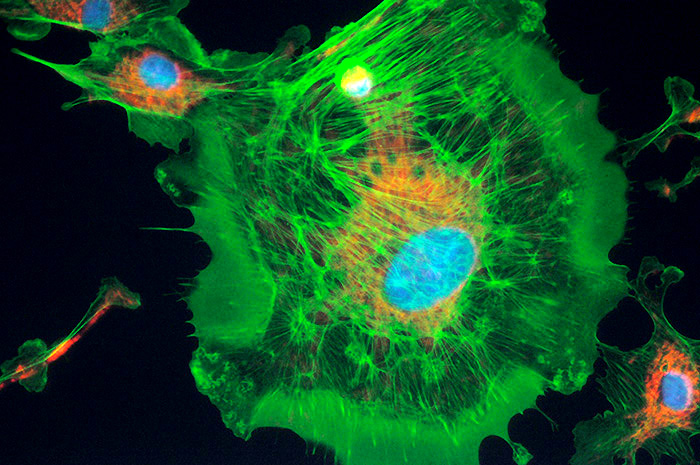
Three merged images using UV, blue and green excitation within a Nikon D300 DSLR. A 100W quartz halogen lamp was used for the fluorescence not the mercury lamp. Individual exposures 5 - 10 secs at ISO800. Objective 40x. There was sufficient near UV to show the nucleii. The exposures for each colour channel can be set as desired. After the third exposure the camera automerges to create a new image (retaining the masters). The camera has an option to merge images with or without an exposure 'gain' compensation. e.g. a third of exposure for each of the three images but advises for darkground images not to do this.
Comments to date
The complementary BPAE slide from studies to date is proving to be a superb slide for a variety of studies. It is a demanding test for darkfield but very rewarding. It also serves as an excellent reference slide for setting up a fluorescent microscope.
Comments to the author David Walker are welcomed.
Acknowledgements:
Thank you to the administrators of the Nikon Small World and Olympus Bioscapes competition for generously supplying such a splendid slide.
Thank you also to Walter Piorkowski for sharing his striking darkfield studies of the BPAE slide in the Amateurmicrography.net forum and for permission to link to the forum messages. His work provided a fascinating lead for my own darkfield studies.
Thank you to 'Tardigrade37' on the Amateurmicrography.net forum for the Cytoseal RI data.
Thank you to Mike Gibson for sending his own imagery and studies, shared with permission in the article.