|
No Formalin, No Mercury, New Fixatives:
(Part 1)
|
|
|

|
No Formalin, No Mercury, New Fixatives:
(Part 1)
|
|
|
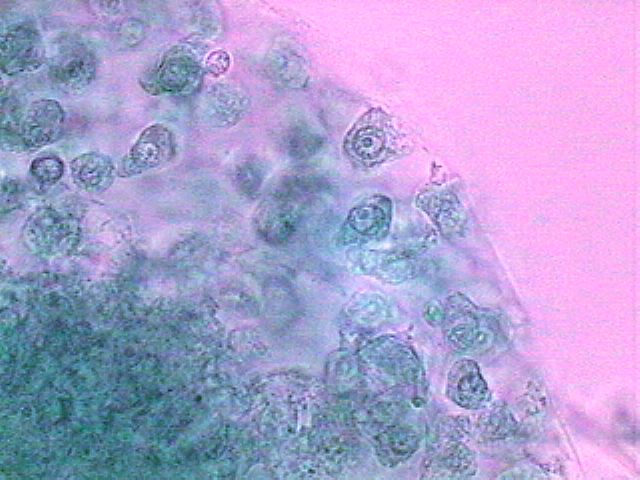
The picture above is from a spherical cluster of cells, not identifiable as any organism that I know, with a nucleus of animal characteristics and amoeboid cytoplasm, found in a preparation of material fixed with GALA 60. A very small drop of “brilliant blue” gives them this color. Click on the image for a bigger version of the picture.
INTRODUCTION
A few years ago, began the tendency to highlight the
dangers of many substances normally used in microscopy for
more than one century. Successively they were denounced:
formalin, glutaraldehyde, mercuric
dichloride, picric acid,
chloral hydrate, phenol, thymol,
gentian violet, Bengal Rose, xylol, benzol, toluol,
(xylene, benzene, toluene) etc.
etc...
The short preceding list includes the ingredients for
almost all the traditional formulas for microscopical
reagents including fixatives, dyes, clearing agents and
mountants.
Even in a world where poisons and dangerous
substances invade the streets and houses (see
this
list of dangerous household
substances
), and for those of us who must live in the cities
where we are surrounded by the smog and the pollutant
gases emitted by cars (whose principal components are the
famous triad of BTX (benzene,
toluene, and xylene), it is certainly
understandable to try to defend the health of the
laboratory technicians, who daily spend 8 hours or more,
subjected to the action of vapors from all these dangerous
substances.
As a measure to limit the use of dangerous reagents,
the industrial producers sell only to wholesalers, and the
latter only to large distributors, which in turn sell only
to professional institutions. Minimum quantities to be
ordered (excessive for an amateur) and the high prices,
dissuade enthusiasts from buying.
It is obvious that, in parallel, these rigorous
preventive measures have other
objectives.
When I was 15 years old and I made my first
microscopic observations of micro-invertebrates, I had
gone to the pharmacy on the corner, two blocks from my
address, and very naively I had requested 100 milliliters
of Rousselet's Liquor,
according to the formula provided by
Langeron in his
"Précis de Microscopie", in
those times my Bible.
This formula, which my pharmacist provided without
the least objection, includes 1 g of
cocaine hydrochloride!
Moreover its price was not excessive!
For my smears of protozoa my favorite formula was
Schaudinn’s fixative, 2/3 of
which consisted of a saturated solution of
mercuric dichloride; and I
fixed the samples of trematodes which I extracted from
frogs in a park pond, with
Bouin’s, made with
picric acid and formalin,
always according to the indications of my
Bible.
To make them more transparent and to better see their
anatomy I employed
chloralphenol and
carbolxylol, both with
phenol. I employed canada
balsam dissolved in xylol for
the preparation.
Only one minute of thought reminds us that
mercury is really terribly
dangerous (remember Minamata in Japan). Even the
thermometers are filled today with colored alcohol.
Picric acid, and picrates and
to a lesser degree, chloral hydrate,
and phenol, are all usable to prepare homemade
explosives, and cocaine is a
substance causing a terrible dependence. Solvents with
hydrocarbons also cause dependence. They are favorites
among poor young people.
The protection of the histologists is thus related to
the design of a wider social
protection.
Consequently, by legitimate or other reasons, we are
subjected to limitations which require that by our spirit
of invention we search for new solutions. Which could be
made without prohibited substances, and be accessible to
no matter whom, amateur microscopist or student, in
suitable small quantities.
Personally I only know one English commercial
supplier which supports the point of view of the
non-professional microscopists, but, although it declares
that it can send its products everywhere, in many
countries legal mail rules, taxes, and even the economic
situation, prevent us from benefiting from this help. (See
on the main site index the link to reach
Brunel.)
I began a series of articles, already published or
which will be published in Micscape Magazine, which examines
the possibilities of amateurs for homemade
preparations.
1) of mounting media, (Micscape Magazine,
December
2002
,
January
2003
, March
2003
, April
2003
, May
2003)
2) of fixatives for various uses,
3) of nuclear and cytoplasmic dyes.
|
One moment of
thought. Nothing can equal the
beauty of our critters when they are alive.
Photomicrography and drawing are the tools of
choice to document their characteristics. One resorts to fixing (and staining also) only to seek details which are not visible in living organisms. Many protist nucleii have a characteristic form, even specific some times (e.g. Euplotes , Ciliates, Hypotricha) and it is very difficult to detect them in the transparent and mobile animal. Undulipodia (the modern name for cilia and flagella) have too much rapid movement for one to appreciate in detail their disposition on the living organism. Moreover, many organs (of the rotifers for example) change much of their form in the living organisms. If in your collections there are some of the smaller larger invertebrates, like entomostracans or acarina, and many other micro-arthropods, by fixing them you can make a collection of samples to be examined a little later, when you have the occasion and the possibility to do it. And certainly, for those who have the chance to have a microtome at their disposal, even if it is borrowed from a school or an academic friend, fixing opens the possibility to investigate the histological structure of the animals. |
|
sample, ml |
4 |
8 |
12 |
16 |
20 |
|
GALA 60 ml |
1 |
2 |
3 |
4 |
5 |
|
|
|
|
The previous ciliate image,
the
Bosmina
at
left, and the
Volvox
at
right are all pictures of materials fixed in
GALA 20 by Christian Colin who kindly allows
their inclusion here. |
|
Lactic
Acid...........................1
ml
Acetic acid
5%.................... 6
ml
Water................................
93 ml
Copper sulfate……….......... 5
g
|
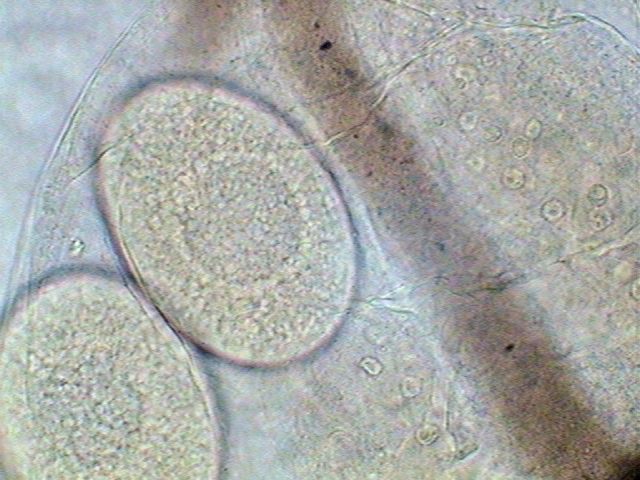
Egg of daphnid, in the brood pouch of a Cladocera. Nuclei of adjacent tissues are seen. Click to increase. |
|
|
|
| Above there are a small cluster of algae (diatoms, at top right: cyanobacteria, below left and chlorophyta in the center, one month after fixing with the LC and mounted in pure glycerin. | Above is the epithelium of the underside of a leaf, (at 1000X) fixed with LC and mounted in fructose; one sees the chloroplasts of the guard cells of a stomata and the nucleus of an epithelial cell (at left). Chlorophyll can still be recognized after one month. |
|
|
|
| Above is a photo taken with homogeneous immersion of the objective (x1000) showing the edge of the egg bag of a copepod. The subjects of the picture are certainly the very small colonial ciliates fixed on eggs by a rigid stalk. | Above one sees the cells of tissues under the carapace of the copepod. Most probably they are ovocytes developing in the ovary. |
|
Brachionus
bidentata
. Click to increase |
Cephallodela
sp. |
Platyas
quadricornis
Click to increase |
|
The images of
Brachionus
and
Platyas
were composited with
CombineZ
software. |
||
0,2 gram………. copper
nitrate
0,3 gram ……... copper
dichloride
1 gram……… acetic acid
100 ml……….… water
|
|
|
|
A daphnid with its brood poach full of
eggs and mounted in PVA-G. Contrary to what I advise, this sample was preserved (as a test) in the same LC for more than two months before making the slide. It is in good condition, but I think it is good not to try the devil. |
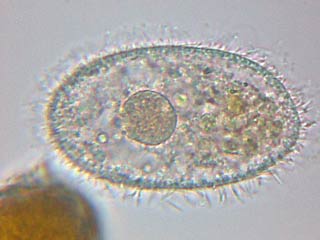
This final image is of
course
Vorticella
fixed in LC with its semi-contracted stalk and its
horseshoe nucleus. I needed to amalgamate 3 pictures to appropriately show the flexibility of the stalk. |
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy UK web site at Microscopy-UK