 |
I - MOUNTING MICROSCOPIC SUBJECTS. Part 3c - The mixed formulae
|
|
|
 |
I - MOUNTING MICROSCOPIC SUBJECTS. Part 3c - The mixed formulae
|
|
|
All pictures originally captured at 640 x 480 pixels. Many of them are amalgamated with CombineZ. Click on an image to view the master. The title image is the air tube of a mosquito larva mounted in gum arabic - fructose medium.
Editor's note: Part 3 of this series will be published in three monthly sections. They will be interlinked as they are published.
Part 3a: Introduction; fructoglycerol and modified Brun medium as mountants.
Part 3b: PVA-lactic acid and PVA-glycerol mountants.
Part 3c below: von Apathy's original formula and Lille's medium as mountants. Gum arabic fructose, glycerinated gum and glycerinated lactic acid mountants.
GUM
ARABIC MEDIA
APT.-
von Apathy original formula
Two references on the web claims an RI = 1.52. I think one is an error, the other a copy of the first. All my four old references state a credible 1.42 RI even with the fructose modification discussed later.
gum
arabic...................…........50 g
refined
sugar...........................50 g
water.......................................40
ml
antiseptic..............................10
ml**
The recipe states that you must use clean drops (stones) of gum arabic. Mix sugar and water and add the gum last. Put in a sealed jar. Completely dissolve it on a stove at 40ºC (from a few hours to days) stirring only occasionally and slowly. Or use a water bath at 60ºC for a more rapid dissolution. Take care not to include air bubbles. Finish adding the antiseptic. The antiseptic is needed because without it the medium develops fungal infections in less than 2 weeks. The amount of water can be raised to 90 ml.
This is the general method to prepare the gum solutions recorded in the literature. If it is thicker than you want, or if you want to filter the liquid, dilute with enough water. After filtering you must concentrate the medium by evaporating the water on a stove.
It
is a safe and cheap formula. The problems are to get the drops of arabic
gum, the difficulty and the time needed to dissolve it…and the frequently
reported problem of sugar crystallization. When I was a biology student
all the “lipid tissue” slides in our histology lab were stained with Sudan
III, and mounted in Apathy’s medium. Many of the slides were more than
five years old yet I've never seen a crystallized one. But…

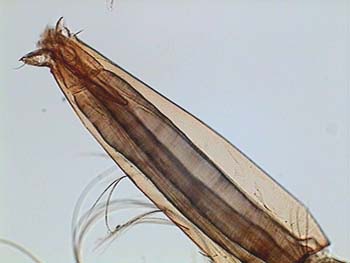
APT, fly wing-1 |

ATP, fly wing-2 |

APT, Diaptomus male antenna |
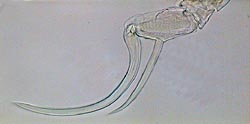
APT, Diaptomus 5th male leg |
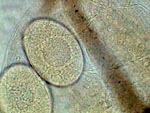
APT, daphnia eggs |
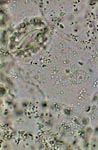
APT, epithelial cell of leaf underside |
Notes on the quality of the gum based on my trials within the last hour! I insisted with my apothecary on the quality I required of the Arabic gum. He consulted with his suppliers and assured me that the grade he was offering was really Arabic gum, powdered, but pure. I remembered my old days as a young scholar when I bought the powdered Arabic gum and put it in water, only to finish with a sphere of moistened compacted powder enveloped by a shiny bubble of air, and all that work to break it and dissolve (over many hours) my paper adhesive.
I
was near to refusing the offer when I remembered my “cocoa trick”. My powdered
cocoa, like the Arabic gum with the water, refuse to mix with my cold milk.
But I have a trick, you know, I mix the cocoa with the sugar, and later
I add the milk. And this works. So I bought some gum. Try this technique:
1) In a 30 or 50 ml flask, put 1 part powdered gum, and 1 part sucrose. Seal and agitate thoroughly to obtain a dry homogeneous mixture.von Apathy’s medium isn't compatible with normal histological stains. The pH of the medium is near 4.0 (highly acidic) so stains fade or bleed into the medium. If you only mount unstained materials, or you intend to preserve your slide for only a few weeks, this is not a concern for you. But some modifications claim a better behavior for the formula. Three alternative added chemicals were proposed: potassium acetate (30 to 50 g added to the formula), calcium chloride (10 g) or even sodium chloride, the common table salt, (10 g) It seems that these additions raise the pH to near 7.0
2) Slowly add portions of the water, mixed with the antiseptic. Any sugar crystals act as micro tunnels that carry the water down the mix. Carefully move the powder with a needle if it stops running.
3) When all is wetted (it took one minute) leave alone for a time (4-6 hours) the glutinous mass, or put the flask in a bath of water at 60ºC (or a little higher).
4) Presto! You have your gum arabic solution made in five minutes of not so hard work, and all you need now is to allow some time for all the dirt to precipitate to the bottom (perhaps overnight).
5) Decant the supernatant liquid and adjust the density to suit your preferences.
Mount
using the technique proposed for Karo. It is not necessary to seal the
coverslips. But I do.
|
** The original formulas for gum arabic or gelatin media always include an antiseptic: thymol crystals (one crystal), phenol (1 g), Mertiolate (10 ml), etc. All are banned as they are either difficult to obtain or are very toxic. Remember that this additive functions just as an antiseptic so you can even eliminate it without affecting the mountant's performance, and take the risk (most improbable) of a bacterial or fungal infection of your slide, as M. Cairn and J.M. Cavanihac had done. Modern alternatives could be Benzalconium chloride, Listerine, sorbitol, or potassium benzoate. • As Mertiolate contains mercury bichloride it was banned, and the suppliers changed the formula of the product. For a long time a solution of Benzalconium chloride has been sold under the trade mark name of Mertiolate. It was even colored red as the original product was, which made it unusable as an antiseptic in this formula. Now one can find white reagents under the Mertiolate or Benzalconium Chloride name. When you mix it in the formula you could be worried by the milky cloud it forms. Continue to stir carefully until the mixture becomes clear again. • Listerine is a mouth washing solution. Its formula is composed of several disinfectant ingredients that are also used as clearing agents in microscopy. The old formulation had the toxic phenol in its ingredients. It is eliminated in the modern formulation. The published composition is: menthol..........................42.5
mg
The Listerine I get has a slight blue green color (turquoise, says my wife),
but in the used quantities this is not a problem at all.
|
LMM.- Lillie medium.- RI = 1.43
I give you one of Lillie’s modifications of von Apathy’s formula. If you use the powdered gum arabic, also use the fructose as crystals. Add fructose and potassium acetate to the powdered gum. Mix well; add the water and the antiseptic.
gum
arabic (powder)..…………...50 g
fructose
(crystals)……….……….50 g
potassium
acetate…………….….50 g
water………………………………..90
ml
antiseptic……………………...…...10
ml
It
has a good refractive index and a pH of 6.7. Most commentaries are
as for upper formulas.
MY GUM ARABIC FORMULAE
As
I can’t find drops of gum arabic (and I had not have yet remembered my
cocoa trick) I made another trip to the art supply store, and returned
with a medium syrupy solution of it as sold for artists to mix their watercolors
and for finishing their art works. These formulae are provided as examples
for you to develop your own. Some of them do not contain sugar, so you
can’t do the old trick. You need the commercial solution.
GAF.- Gum Arabic-Fructose medium (RI probably around 1.42)
Gum
arabic solution.........................................30 ml*
(Commercial
Karo “Clear” (for babies)) ..........30 ml **
*If the commercial solution contains excess liquid, concentrate it by evaporation.
I
omit the antiseptic because for obvious reasons the commercial solution
must have one.
Those
concerned about bleeding of histological dyes can include 15 g of potassium
acetate (or try the cheaper and ubiquitous sodium chloride…. 1 levelled
teaspoonful) to raise the pH.
The fructose substitution for the sugar lowers the danger of crystallization. It is a worthwhile and safe precaution to seal the coverslip to stop evaporation.
Mount
directly from water and as if it were pure Karo. File horizontally.
Commentaries.-
This
is obviously my version of the Lillie’s medium. The RI, and the appearance
of the mounted subjects are good, and it dries fast (in my climate). As
it is somewhat liquid (at least in my flask) carefully estimate the drop
size to avoid excessive mountant overflow. I challenge you to adapt this
formula using powdered arabic gum and fructose crystals.
GG.- Glycerinated Gum.- (RI about 1.44)
This is a formula in the style of those proposed by Farrant, Lillie, and Dahl.
gum
arabic solution..…..………….30 ml
sugar…………………………...…....30
g
glycerin………………………...…....30
ml
antiseptic………………….….…......10%
If
you want, you can add sodium chloride (10 g) or 20 g of potassium acetate.
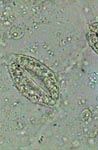
GG, epithelial cell of leaf underside |

GG, fly wing-1 |

GG, fly wing-2 |
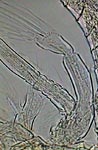
GG, female Diaptomus antenna |

GG, Diaptomus ovisac |
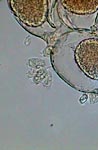
GG, epizoics on Vorticella |
GLG.- Glycerinated lactic gum
lactic
acid………………………...25 ml
gum
arabic..………………….…..40 ml
glycerin………………….………..20
ml
Karo*………….………………..…10
ml
*or Larry’s fructose syrup
The
above is my recommendation as a substitute for the Hoyer, Berlesse, de
Faure and other chloral hydrate based formulae. It is recommended for botanical
sections cut with a microtome, including stained ones, or for arthropods
or other subjects cleared in lacto-glycerol. Of course this and the PVA-L
are the appropriate mediums for chitinized arthropods cleaned of their
soft parts with potassium hydroxide or sodium hypochlorite. Small and soft
subjects can be overcleared. Try the lactic
acid methylene blue staining (one that even
I have not tried).

GLG, head of mosquito larva |

GLG, pecten in airtube of mosquito larva |

GLG, airtube of mosquito larva |
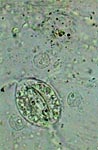
GLG, epithelial cell of leaf underside |

GLG, Diaptomus male antenna |

GLG, fly wing-1 |

GLG, fly wing-2 |
In my bottle this formula is highly fluid, and I first suspected it was of little use. But it is really an easy to use media. Drying time depends on the climate I think; it took much more time here in moist weather. If you mount directly some more or less opaque objects, they clear slowly in 8 to 12 hours. Muscles can be made invisible in the medium term. Generally this is the effect that users of this type of formula are searching for. Additionally you can clear your subjects, before mounting, with lactoglycerol, or lactic acid, if they are really dark.
The
GLG gives results very similar to the PVA-L. Probably the degree of clearing
imparted by each formula could be regulated by changing the quantity of
lactic acid included. It dries firm in some hours, but it is a good idea
to seal the coverslips.
Mounting in gum arabic media
With an appropriate tool pass the objects from water or an aqueous medium to the slide. With an absorbent paper eliminate all the water you can. Add a very tiny drop of medium, position your subjects as desired with the aid of two needles. Lower over them a carefully estimated drop of the gum media. It is better if it is a little undersized. Apply with care the cover slip. When you apply the weight the gum must reach the borders. You can hurry up the drying process by applying heat with an electric bulb, or with the microwave oven, as explained before.
If
you live in a climate with 30% Relative Humidity or less this is all you
need. But over 40, 50% RH you need to seal the coverslip.
| As this article has run for much longer that I intended, I leave for a future article the discussion of the jelly formulations and of which of the 18 fixatives discussed in this series must be used and when. |
Comments to the author, Walter Dioni, are welcomed.
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy UK web site at Microscopy-UK