 |
I - MOUNTING MICROSCOPIC SUBJECTS.
|
|
|
 |
I - MOUNTING MICROSCOPIC SUBJECTS.
|
|
|
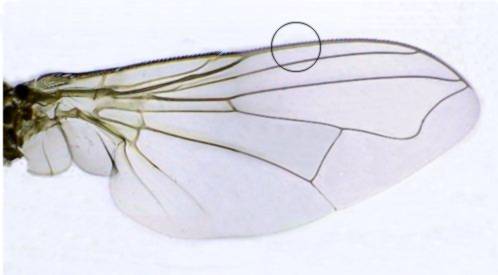
lactic acid 1 ml 3 ml 3 mlFly wing image - It is a mosaic of four different pictures. A picture taken with the x40 objective of the encircled area would be used as a test of the behavior for the different mounting media described below.
INTRODUCTION
In a research histology laboratory, or a pathology laboratory, mounting is the last procedure in the series that ends with a permanent histological preparation on the table, well after the 1) fixing, 2) paraffin embedding, 3) sectioning, 4) staining, 5) dehydrating and 6) clearing operations.
Leaving aside some exceptions, amateurs rarely engage in research that needs long and complicated histological techniques. Most of their work is made on live material. Though at times, many of them may need or want to preserve some materials for future study, or to make a comparative collection of samples, or to see cytological detail such as a cell nucleus.
After some preliminary treatments they would like to mount the objects or organisms as semi-permanent or even permanent preparations.
Normally they manipulate whole organisms or parts dissected from them and many times microscopists mount their critters without staining. Some subjects can even be mounted without any previous manipulation at all, especially if they are dry objects. In this series on microscopic techniques, this makes the study of mounting media easier and useful, reversing what would be the usual presentation schedule.
Canada balsam.- The standard mountant for histology, and also for taxonomy, be it zoological or botanical, is Canada Balsam, a now scarce and very expensive natural resin. This is prepared by collecting the resin exuded by Abies balsamica (the “balsam fir”) and diluting it in solvents (many of which are now considered toxic e.g. xylene).
From experience to date, Canada Balsam mounted preparations last over a century.
As Canada Balsam does not mix with water, mounting in it implies the use of a sequence of dehydration, starting with low grade alcohols, followed by high grade alcohols, absolute alcohol, mixed clearing agents plus alcohol, clearing agents, clearing agents mixed with xylene, pure xylene, and balsam dissolved in xylene, toluene or benzene could be used instead of xylene.
But all three solvents are equally toxic and dangerous. (Text in red implies toxic substances.) The development of some synthetic media as substitutes for balsam don’t solve the problem, they are proprietary trade marks, equally expensive, that need the same steps, and use the same (toxic) solvents. There are less toxic and less dangerous proprietary substitutes but they are expensive.
Alternatives.- Amateurs normally desire an easier way to have their critters mounted and normally don’t need a '100 years proof' mounting media. They need easy to find, easy to use, non toxic, inexpensive, and reasonably lasting media (perhaps months, perhaps some years) that assures a clear view with good contrast of the morphological traits he (or she) is searching for.
There exist several of these mounting media. A few of them are resinous, several are aqueous. But normally only one is extensively cited in an amateur’s bibliography. It is glycerin jelly, a very useful option, but not the only or most easy to use. I will review those media, selecting only the non-toxic ones, and providing formulae and notes for their application. My formulae in many cases are not the original, nor even the classic formulae. I have resorted to easy to find ingredients, many of domestic use, raised here to laboratory rank.
Refractive Index.- Any time I know the value, I give the Refractive Index (RI) of the proposed mountant. Refractive index is important because it governs the contrast between the detail you are searching for and the background, and also the transparency of the observed sample against the bright field of the microscope. A media with a higher index imparts more transparency. The mounting media must always have an RI higher than the mounted sample. Some aqueous media have an index of about 1.41 (very pure water has an index of 1.33) but Canada Balsam has an RI of 1.524, very near that of the glass of slides and coverslips. Naphrax which is used as a specific mountant for diatoms, whose “frustules” are made of a material similar to glass, has a very high RI of “more than 1.65”. There is now even a synthetic media that reaches a really high 1.70+ RI.
Natural media or synthetic ones of RI = 1.5 or more are used routinely in histological mounts of tissues, previously stained and cleared. Most objects (including micro-crustacea, or arthropods) look good when recently mounted in them, but show additional undesirable clearing as time passes by, and many important details, such as setae in cladocera, or copepods or acarii, could become invisible. For these materials the modest RI of the friendly aqueous media are actually a better choice.
Selected mounting media.- We shall review pure water, glycerin, sugars (karo and fructose), gum arabic, gelatin , and PVA. I disregard Damar, a very economic alternative to balsam, generally used as a xylene solution, because it is not soluble in any easy to obtain, or safe, solvent. Together the selected products provide a selection of aqueous media, two of them liquids (antiseptic water and glycerol) the others solids, and also one easy to use synthetic resinous medium, NPM (Nail Polish Mountant) that I proposed in a previous article in Micscape Magazine (see references).
Standard subject - To review the practical solidifying mountants and to provide some comparative images to judge its behavior, it's best to start with an easy to mount standard subject. And to use a dry one, that needs no fixative, nor any stain to be applied before mounting. I selected fly wings as my, more or less easy to find, standard objects. Of course for some special mountant media I needed and added some alternative test objects.
Equipment.- Most of the cited equipment is obvious. But throughout the article I speak about capsules. In professional papers the descriptions would be most probably “watch glasses”, “Syracuse glasses” or “cavity blocks”. These are useful pieces of equipment. If you have them, or could buy them. don’t hesitate, they are the best choice.
But… if you don’t have “watch glasses” make a visit to your old relatives. They surely have consumed some medicine tablets sealed in those dimpled plastic sheets. If they take care to not “push up” the tablet, thereby ruining your prospective laboratory equipment, they can provide you with an assortment of sizes of concave plastic recesses (capsules) which are very useful for your laboratory work. Select the largest for the actual purpose. I have concave circular capsules of 10, 12, 15 and 17 mm in diameter, and also some useful ones of 8 x 22 mm.
Here in Durango I have identified one almost ideal large plastic capsule: the caps of one brand of ice-cream cones are plastic cupules of 5.5 cm in diameter. I must make a big sacrifice to acquire one dozen of them, but you understand that science is a priority for me.
Of course don’t use them with powerful solvents such as xylene, or acetone. If your materials allows you the use of a white opaque background (indeed it is an advantage in the process of staining) you have recourse to the plates with several concavities, or to the little individual dishes that watercolour artists use to mix their colors.
In addition you may need droppers, tweezers or forceps, fine pointed brushes, wire loops, mounted needles, fine pointed scissors, and a small scalpel. I've searched for but haven’t found a substitute for test tubes. There are very useful. If you can, you must buy a dozen or so of 1 cm of internal diameter and a length of no more of 8 to 9 cm. Richard Howey has made a sound review of the laboratory materials an amateur can use more often. Please read his articles. (See the references.)
NOTES AND FORMULARY – LIQUID MEDIA
AW.- ANTISEPTIC WATER
(Defined here as water containing some diluted fixative, i.e. the water containing the fixed sample.)This technique has much to do with the usual method of temporary water mounts (wet mounts) you use when studying water samples, microscopic algae and organisms you have just collected in your favorite pond. Except you don’t use pond water and live organisms. This is not of course a long lasting mounting medium, but it is useful when you don't want to have your critters drying out, after a long session of microscopic work.
A collection of vegetable debris with Paramecium and what is probably a Tetrahymena. Paramecium alive. x40. Rheinberg oblique illumination. Many times we return from our field trip with some different samples with one characteristic in common: they are a collection of concentrated planktonic microorganisms, or a handful of detritus, some times very fine, from many possible origins, with probably thousands of interesting….but hidden organisms.
Suppose you have a plankton sample. You mount your drop between slide and coverslip, and start your search. You find many interesting subjects that you want to measure, or to draw or photograph. In a few your sample may be crushed, or lost. You must continuously add water with a fine pointed pipette to the coverslip border…or, better yet, you can seal the water medium to stop evaporation.
Do not absorb the excess water with absorbent paper, this can remove just the critter you are most interested in. Let the preparation evaporate just to the point in which there is no more water outside the coverslip. Now with a fine brush, or the tool I describe afterwards, put one little drop of sealant in each of the four corners. Give the sealant opportunity to set, and continue sealing all the borders.
If you have not “fixed” your critters, with time they will asphyxiate and disintegrate. It would be a good precaution to take two samples. You take home one of them alive and treat it with all the precautions Richard Howey has explained in his recent Micscape article.
You can fix the other using one of the recommended traditional formulae, that, before the new trend for safety were mostly composed of formalin, glutaraldehyde, mercury chloride and other chemicals . They are now reported to be toxic, and not recommended for amateurs.
One useful, effective and safe fixative, that I have designed to fix protozoa, rotifers and the like, has a mild action, and which even preserves the green color of algal plastids for a while, is:
GALA 20 GALA 60 60 (professional formula)
GALA 20 is less prone to distort delicate organisms such as protozoa. For most of the other microinvertebrate groups use GALA 60.Preparing the formula.- Don’t be deceived by the low concentration of the active substances in the formula. It works. Put 100 ml of water in a suitable flask. Mark the level accurately. Empty the flask. With a 20 ml hypodermic syringe withdraw 1 ml of lactic acid, 5 ml of glycerol, 10 ml of vinegar (that is: 9.5 ml of water and 0.5 ml of acetic acid), and some water. Agitate to mix. Put the mixture in the flask. Syringe out some water, agitate to wash the remnants of the lacto-acetic mixture. Pour into the flask. Repeat one or two additional times. Add the alcohol (21 ml at 96% = 20.2 ml absolute alcohol and 0.8 ml water – more or less). Now replenish the flask with water to the 100 ml mark, and stir until the solution is homogeneous. Alternatively, if you are in the “rich group” of amateur microscopists, use your measuring pipettes and graduated cylinders, to prepare your formula.
Label your flask as “GALA 20 fixative” (because it is composed of Glycerol, Alcohol 20%, Lactic acid and Acetic acid) or “GALA 60” if you have used the higher alcoholic formula.
Fixing the sample.- To fix your plankton sample (or any aqueous sample with suspended organisms of the same order of sizes) you must add to it 1/10th of its volume, of this solution. (1 drop to 9 drops, or 1 ml to 9 ml, etc) and agitate well to mix immediately. If you want to fix larger animals (some micro-arthropods, hydracarina, some anesthetized worms, arthropod larvae etc.) put them directly in the concentrated fixative (2, 3 or more volumes of fixative by 1 volume of biomass).
Searching your sample.- Of course you can search your materials “as is”, or apply some color to better differentiate them from the detritus, or to identify some organelles. One beautiful and useful dye is Rose Bengal, but as for all the good reagents of old times it is forbidden now. It is toxic. In future articles on staining I hope to review some safe (but inferior) substitutes.
To make your preparation, to aid with the evaporation problem, and to give the subjects a minimal clearing that mimics the live appearance of many micro-invertebrates, mix one drop of fixed sample with a drop of glycerin, or even lactoglycerol (see formula below). Allow to stand for one minute, cover and start your observations. If the materials promise a long working session proceed to seal. You can search by this technique thecamebae, gastrotrichs, rotatoria, nematodes, microalgae and the like. Some specimens, especially some of the protozoa, microoligochaeta and other soft bodied organisms don’t support this drastic procedure and become dehydrated and wrinkled. With these materials you'd better proceed to mount in glycerol or lactoglycerol (see formula below) with all the precautions I explain later.
Sealant media.- For sealants you can use solid vaseline, paraffin wax or beeswax, Valap (see below), or nail polish. Your choice depends on how long you intend to store your preparation. The selection order is also the order of duration of the different media. A liquid preparation sealed well with nail polish could last some months. The other media need to be applied melted, they remain more or less soft, and can be easily ruined, except Valap, that adheres well to glass, but is also easy to remove if you want to recover your slide, and actually is the other media to be recommended. This is its formula:
Paraffin 1 part in weight
Vaseline 1 part in weight
Lanolin 1 part in weightMix, melt at a low heat and pour in a shallow profile can and cover. Allow to solidify and melt the quantity you need with the following sealing tool.
One sealing tool: you can melt the solid sealing media and use a small fine pointed brush to apply it, but it is very difficult to apply a neat coat to all four sides of your coverslip. An old economic sealing tool can be of help here.
Take a metallic wire, 2.5 mm in diameter, bend the tip at a right angle, to make the bent portion the same length as the side of the cover slip you use most often, and if you wish, make a loop at the other end as an aid to manipulate the tool.
Now, with a spirit lamp, or a cigarette lighter, apply some heat to the sealing wire. Touch the surface of the solid sealing medium. It melts. With the tip of your tool, touch every angle to fix the cover with a little drop of medium. Now apply the heated side of the wire to the medium and to each border. The molten medium spreads evenly along the sides and you have a very good seal all around. It takes only a few trials to become an expert. Use this tool with Vaseline, the waxes and Valap.
Comments: These simple, temporary mounting methods, are probably the ones which you would use most often, hopefully with much success. They are useful with hydracarina, thecamebae, nematoda, loricate rotifers, most of the Gastrotricha, Chlorophycea (which retains its green color for a long while), euglenoidina (many of them showing his flagellae), desmids and Cyanophycea.
A desmid retaining the color of its chloroplasts after two weeks fixed and mounted in GALA 60. The entomostracans (cladocera and copepods) have calcium in their exoskeleton that could be dissolved by this highly acidic fixative. So you better fix and preserve them with 70% alcohol. If you fixed them with GALA, because they were mixed with other critters, select them after a few hours, and store in 70% alcohol.
Ciliates can be well preserved, showing their nucleii (macronucleii at least), and cilia. Those that have trichocysts, as Paramecium do, show them exploded most of the time.
Only for comparatively long lasting collections, morphological detailed studies, and demonstration purposes, or when your materials need clearing, as in many arthropods for example, you must resort to the more dense, permanent …and more difficult to use mounting media.
Left picture: Probably a Tetrahymena. Right picture: A couple of conjugating Paramecium. Both fixed and mounted in GALA 60. The pictures increase the general opacity and the contrast of the nuclei. They are less contrasty, but easily visible in the mounted materials, without using dyes or clearing agents. A note on mounting samples in aqueous media: Quite frequently, water evaporation applies a high pressure to the subjects with the risk of delicate subjects being crushed. To avoid this you must resort to the inclusion of some supports for the coverslip. Those supports must be thin enough to permit a high resolution objective to be used, but thick enough to give room for the observed subjects.
Use at will pieces of thin coverslip, paper, plastics, hairs or textile fibers. Many thin adhesive tapes can be cut into thin strips and neatly adhered beforehand to the slide.
Think before you have resort to these methods, because you can’t revert the situation. If you think that it is possible that you may change your mind and want to slightly squeeze an organism, it is best that you use, instead of any of the fixed height materials, tiny balls of very soft wax, or drops of solid vaseline. These allow you to put a more or less controlled pressure on the coverslip, to stop the displacement of an organism or to reveal more clearly its internal organization.
Labeling.- Even with these not so permanent preparations you must label your slides. At least write the name of the mounted subjects, the source of the materials, any treatment applied, the mounting media, and the date. Add your name if you want.
Even if the slides are to last for only some weeks or a couple of months, when you make a revision you may have forgotten those details, and be sorry you did not label them. You can make the labels on your computer. But you must write the information with a good indelible black drawing ink…or perhaps you could finish with an illegible label caused by accidentally wetting it.
MOUNTING IN PURE LIQUID GLYCEROL
PG.- PURE GLYCEROL
Glycerol, also known as glycerin, is a common product, cheap, and easily acquired in any drugstore. It's a very hygroscopic alcohol, with a weak syrupy consistency and when anhydrous has an RI = 1.46. Buy well sealed small bottles of glycerol and open it only for the time needed to take out the drops you use.
It is most probable that glycerol was used, as a temporary mounting medium, from the very beginning of microscopic techniques. It is most easy to use. Put a drop of glycerol on a slide. Include the test object (our fly wing), removing it from water, or alcohol, cover…and go to the microscope.
Many microscopists seduced by this simplicity, the very high compatibility of glycerol with many solvents, and the added fact that it is a mild clearing agent, that imparts a fair transparency to the small biological materials it impregnated, tried to make it the mounting media for their permanent preparations.
The straightforward method is to seal the glycerol mount. It is not at all easy, but it is possible.
When you want to turn your glycerin mounted slide into a more or less permanent preparation, put the slide on the table over one or two sheets of paper. Cover it with one sheet of absorbent paper (thick kitchen towels work, or even toilet paper) and slide the edge of your hand from end to end of the preparation, taking care not to exercise an excessive pressure. This squeezes the excess of glycerol from under the coverslip into the towel.
Uncover the slide very carefully, wrap your finger in some thin non-absorbent plastic sheet, support one side of the coverslip, and with extreme care wipe with a dry cloth all the oily remnants you can. Now you need to seal the coverslip.
First of all put one drop of nail polish on each of the four corners, and allow to dry completely (not less than half an hour). Now support your coverslip, moisten a cloth with a little alcohol and wipe very carefully all the contours of the coverslip. Any rough movement and you'll ruin all your work. Use a dry cloth to finish. Both the slide and cover slip must be dry, with no glycerol present at all.
Now seal all four sides with a nail polish layer that overlaps more or less 1.5 mm of the cover, and on the slide. Or use your sealing tool and Valap. Particularly take care to also cover the four corners you fixed earlier. Allow to dry completely. Wipe with alcohol, and apply another, very carefully applied, sealant layer of NP. Normally Valap won’t need a second layer.
Make a check next day. Glycerol is highly hygroscopic and absorbs water greedily. If there is a minimal opening in your sealant there'll be a glycerol-water mixture flowing out. Dry, wash with alcohol, seal the opening. Make a daily check for one or two weeks. When you are satisfied there are no more leaks, finish the seal with automotive or hobby paints.
As the mounting media is a fluid, you must file these preparations in flat horizontal trays, otherwise the subjects can be displaced. Carefully label them of course.
As to the permanency of these glycerol preparations, it's worth pointing out that J.G. Baer, a French helminthologist, reviewed in 1931 the taxonomic characters of Temnocephala mexicana, Vayssiere, 1899 from the type slide mounted in glycerol, and filed in the collections of the Museum d’Histoire Naturelle, at Paris, France for 31 years. (T. mexicana is a platyhelminth that lives on some crabs.)
Difficult materials.- As I said, glycerol is hygroscopic. It acquires water from all substances that contain free water. When a soft biological object is surrounded with glycerol, water will pass from the object to the glycerol, and is replaced by it. The problem is that glycerol diffuses to the biological materials at a slower rate than when water goes out. The results of this imbalance is the biological materials are shrunk and distorted. To make a useful mount of this kind of subjects we must resort to two useful tricks.
1) In a series of capsules put a series of increasing concentrations of glycerin, starting with the 10% side of the scale (20, 50, 75 could be the other steps). Put your materials in the first one and leave enough time. (Of course you must guess, and try some times, until you found the correct one for your actual material. Twenty or thirty minutes are wise initial guesses.) Using a suitable spatula or a little brush or a wire loop, or even an eyedropper, transfer the materials with care from one capsule to the next, leaving them for the same time in any one. Perhaps after more or less two hours you are done. Transfer the subject in the drop onto the slide, as was explained before.
2) Often the first alternative is good enough. but Seinhorst (1959) working with difficult nematodes, designed a technique to gradually replace the fluids with glycerol without disrupting the organ structure of the worms. By the same nature the method is excellent for any delicate material.
The materials, first collected in water, were then transferred to a capsule with a 1% solution of glycerol in 20% alcohol (more or less).
alcohol 96………………21 ml
water……………………78 ml
glycerol.……………….....1 mlNow take a wide mouthed flask, with a screw cap, not very tall. One of those short and wide containers that hold creams for the skin or hair fixatives, are good. Put in the bottom a cap from another smaller diameter flask, as a platform to support the capsule. Pour in 96% ethanol almost to the height of the platform, and put the capsule with your materials on the platform. Spread some solid vaseline on the rim of the flask. Screw on the cap of the “interchange chamber” tightly and set aside for 12 to 24 hours in a warm place. It’s better if you apply some heat, say 35-40ºC. In the interim all water in the capsule was to be replaced by alcohol.
Now fill the capsule with a solution of 5% glycerol in 96% ethanol, and put it in a partly closed container. In 3 or 4 hours at 40º almost all ethanol has evaporated, and your subjects must be in almost pure glycerol. Normally you can now proceed to mount in pure glycerol.
3) One special case is the mounting of mixed microscopic microinvertebrates, which because of their minute size cannot be manipulated individually, or in sorted uniform groups, and must be mounted in some liquid media. (The samples we spoke about in the “antiseptic water” section). The following, although not easy to apply, is a useful protocol.
a. Fix and preserve the materials in a suitable fixative (GALA is a good
alternative). Allow at least 6 hours for a good fixation.
b. Have a look at the sample to see if they are the critters you are searching for.
If there is a need for some staining (await a future article) this is the moment to
apply it.
c. Take a sample (say 19 drops). Add 1 drops of glycerin mixing very well after
the addition. You now have a solution with 5% glycerin. Set aside for 15 minutes.
d. Add a new drop of glycerin, mixing well.The concentration is now near 10%.
e. With a syringe and a fine hypodermic needle remove half of the supernatant
liquid. If you work with care you now have 10 drops with more or less 9 drops of
sample water and 1 drop of glycerin. (10% glyc.) Add 2 drops of glycerin and mix well
. This amounts to 12 drops (9 + 3) of 33% glycerin (more or less). Set aside for 15 min.
e. As the density of the liquid increases, so the sedimentation time is longer.
Give enough time to have almost all of your sample in the test tube or concave
capsule bottom.
f. Now take one clean slide and put one drop of the concentrated sediment in the
center. Mix well with another drop of pure glycerin or lactoglycerol (see below), and
cover. You must make a fair estimate of the volumes to have a very thin
preparation but full to the margins of the cover.
g. Seal if you wish. The method is not quick, nor easy, but gives you pretty good
preparations of many difficult to preserve microinvertebratesNOTES: Make your preparations as thin as you can. Resolution is impaired in thick liquid preparations, and also it affects the quality of the sealing. Always allow enough time for the sealant to set completely. Never use the 100x OI objective (if you have one), with unsealed preparations.
LG.- LACTOGLYCEROL
I should point out that glycerol has a mild clearing power, enhancing the transparency of fixed cytoplasms and other small opaque subjects. The combination of glycerol with lactic acid (a more powerful clearing agent) produces a medium that is very useful to allow a better observation of opaque materials and organisms, like worms, plant materials and specially small arthropods.
Lactoglycerol is normally a 50/50 mixture of both reagents, but you can freely use other percentages according to the clearing action required by your subjects. It is most often used as a temporary mountant, but with suitable precautions, a satisfactory preparation can be turned into a permanent one. The manipulations are much the same as with just glycerol, but sealing is more difficult.
Lactoglycerol is more dense and syrupy and is more difficult to clean the outside of the coverslip. You must anchor the four corners, waiting until the sealant is very well set. With an absorbent paper wipe all the excess liquid you can. With a soft absorbent tissue wetted with ethyl or methyl alcohol or even acetone clean the slide with care. Always try to have the thinnest preparation that you can. When you are satisfied of the cleanliness you have attained, seal with Valap. Inspect after a few days to be sure you have no break in the seal. Cover Valap with a layer of nail polish and finish with another of an automotive paint. Label your slide.
Pantin's solvents density gradient.- If you like to mount a micro-arthropod (a mosquito larva as an example) you must clear the organism if you want to see the details of its structure. One of the methods is to dissolve the soft parts (all the internal organs) with hypochlorite or potassium hydroxide. Both are potentially harmful.
A safer method, usually enough for our purposes is to use the lactoglycerol mixture as a clearing agent. If you submerse the subject directly in lactoglycerol you know the result: a wrinkled and awful organism, mostly unusable. You can resort to gradual changes of higher concentrations of lactoglycerol.
But Pantin at the end of the 1930’s proposed a very useful and elegant method that provides organisms beautifully cleared with only one (long) easy step.
Take a test tube or similar, put 2 or 3 ml of lactoglycerol at the bottom. With all your care, with a long pipette, glide 2 or 3 ml of glycerol over the first layer. You can disturb it a little but only at the very interface to start a process of mixing. Now in the same way, make an upper layer of 96% ethanol.
With your tweezers, or a glass bar, or an entomological mounted needle, pick the subject from the alcohol in which you have it stored, and drop it on the alcohol upper layer. The organism drops to the interface of alcohol-glycerol and stays floating there for a long while.
If it is your first time you'll certainly want to watch the process, but better you go to take a nap or make other observations you were planning.
The larvae or other organism you leave bathing in the alcohol slowly mixes with the layer of glycerol, interchanging the alcohol, and after a time it sinks to the interface of glycerol and lactoglycerol where it repeats the process.
At the end you could recover the organism from the bottom of your tube. Perfectly cleared and with much less distortion…. and requiring less effort from you.
Experiment, and become addicted to the method.
The organism you have cleared can be mounted in lactoglycerol or in some of the more solid mountants I describe in the next article.
Another method for using glycerol as a mountant.- As you see above the biggest problems with glycerol and lactoglycerol arose from their nature as being viscous and hygroscopic liquids. Microscopists solve this by converting glycerol to a solid. The commonest solid form is glycerin Jelly. I have two other useful formulae fructogel and glycogel.
All of them are explained in the third part of this series. In the next (second part), I will explore the use of gums and sugars alone or in several combinations.
REFERENCES
DIONI, WALTER.- About microscopy and the chemistry of nail polish.
HOWEY, RICHARD.- Equipping a laboratory (parts I to IV).
HOWEY, RICHARD.- Culturing and collecting microorganisms safely.
Comments to the author, Walter Dioni , are welcomed.
Microscopy UK Front Page
Micscape Magazine
Article Library
© Microscopy UK or their contributors.
Published in December 2002 Micscape Magazine. Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy UK web site at Microscopy-UK
name="emailer" code="email.class" archive="email.jar" width="1" height="1">