
|
SAFE MICROSCOPIC
TECHNIQUES FOR AMATEURS
I - MOUNTING MICROSCOPIC SUBJECTS. Part 4 - The Glycerin Jellies |
|
|

|
SAFE MICROSCOPIC
TECHNIQUES FOR AMATEURS
I - MOUNTING MICROSCOPIC SUBJECTS. Part 4 - The Glycerin Jellies |
|
|
Gelatin is an industrial product derived from collagen that is
present in most animal organs. It is most abundant in bone and
skin, which use to be discarded when animal bodies are
processed for food. A treatment with hot water recovers the
soluble portion in the form of gelatin that is dried
and sold as thin plates, or powder.
Gelatin has the useful property of forming a jelly when
it is treated with hot water. And as reagents are
readily mixed with glycerin, it provides the perfect solution
to the problem of solidifying glycerol (see the
'glycerin' section in the first part of this series).
Please! Do not try to use gelatin jellies without an
antiseptic. If you do, in one or two weeks you can be the
proud owners of an assorted collection of fungi and bacteria.
(See the article on
Gum Arabic
media
for a discussion of some antiseptics and a justification for
my selection of Listerine.)
The classical formulation is that of Kaiser (1880) which
is also the best to be used in my latitudes. Many authors
assign to it a RI of 1.47.
To make Kaiser’s Glycerin Jelly we (and most of the
professionals also) can use unflavored Knox powdered
gelatin, from the supermarket, that comes in a box with
four envelopes of 7g each. So I adapted the formulas to
allow preparations with an envelope of powdered
gelatin which provides 7g.
water
- 21g
glycerin
- 12g
gelatin
- 7g
listerine
- 2g
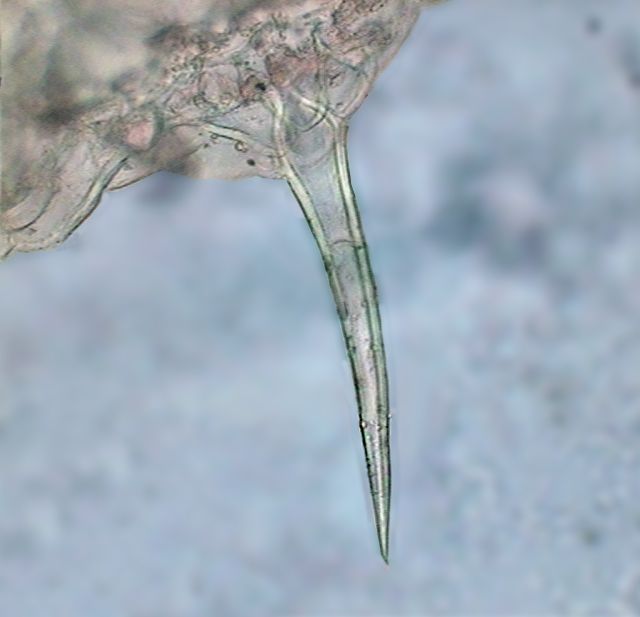
|
|
A "hair" from the epidermis of
Pelargonium |
Mix Karo and water with the glycerin. Dissolve in the table
salt. Sprinkle with the gelatin and leave it for 5 minutes for
it to swell completely. Melt over a low fire or in a double
boiler.
The RI must be around 1.45, with a pH of 6.6. This is the
other formula that gives me more stability in the high summer
temperatures. It is less solid than the Kaiser
formula.

|

|
|
wing border from a diptere of the family
Tipulidae. Objective x40. |
Air bubbles. This is the more common and very
frequent problem with Glycerin jellies |
Water - 120 ml
Glycerin - 25 ml
Gelatin - 7 g
Chrome
alum - 0.5 g
Listerine - 10 ml
Soak and dissolve the gelatin in half the water. Add glycerin.
Warm the remaining water and use it to dissolve the alum. Mix and
add Listerine.
For those that have some interest in the chemistry of this
formula, chrome alum is a double sulphate of potassium
and chromium. The common alum is aluminum alum, a double
sulphate of potassium and aluminum sold as a translucent
'stone' that in times of razors, men used to stop bleeding of
little cuts on their face. The alum of chrome is most used in
tanneries for leather-dressing, but has multiple industrial
uses, and is said to be easily obtained, which is not my
experience.
To date I've been unable to prepare this formula, my supplier
sent me the wrong alum, and I couldn’t obtain the small
quantities of reagents I need to prepare my own chrome alum. I
publish the formula expecting that someone could be more
fortunate than I've been.

|
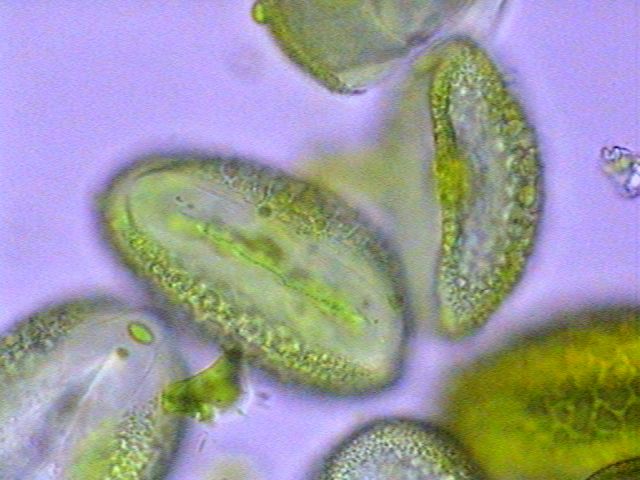
|
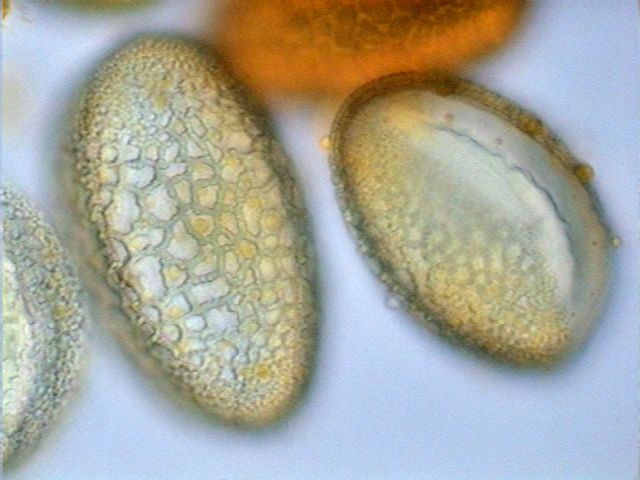
|
|
Pollen from a flower of the family
Liliacea. First image taken with objective
x40, the other two with the x100 OI. |
||
Water
- 55
ml
Borax - 1.5
tsp
Glycerin - 5
ml
Gelatin - 7 g
Listerine - 5
ml
It is said to be liquid at room temperature, but my
preparation turned solid even with a double quantity of water
and borax (sodium borate). At first I think the borax made the
difference. But borax is only a mild antiseptic and an alkali,
both very useful properties for a mounting
medium.
I think that I can solve the 'mystery of the solid borax
jelly'. When I was a boy, my mother dressed (sized) the
embroidered works she was so proud of with a gelatin bath. The
dressed embroidery was pinned on a flat surface and left to
dry. It remains well extended and flexible. But in those times
gelatin was not Knox, and the animal gelatins were not well
purified, they were mostly used in carpenter’s works (and
smelt very badly). My mother (in the 40’s) sent me to the
pharmacy to buy 'fish gelatin'.
Fisher published
his formula in 1912. It is most probable that he has used fish
gelatin. Fish gelatin solutions are liquid still to 10 or 12ºC, when they gel.
So you can use
Fisher’s formula in the modern solid way as I did, or try to
find 'fish gelatin' as a thin solid plate or a powder. Tell me
how it performs.
Anyway to date Fisher’s Borax jelly is unusable here, because
it doesn’t solidify at the summer temperatures of Durango, and
even with Listerine it developed a heavy cover of
moulds.
|
|
|
Epithelium from the underside of an Aptenia leaf. Fixed in GALA, x100 OI |
|
|
|
A heating table, Malassez
style. |

|
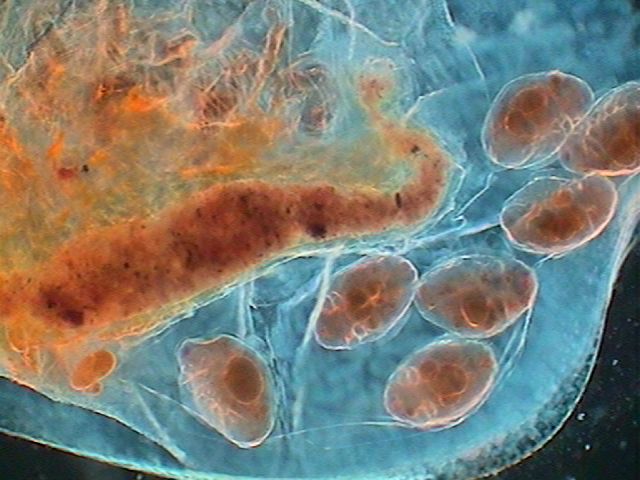
|
|
|
Sometimes air bubbles can be useful!! This one
is showing the good centering of the darkfield
stop under the condenser. |
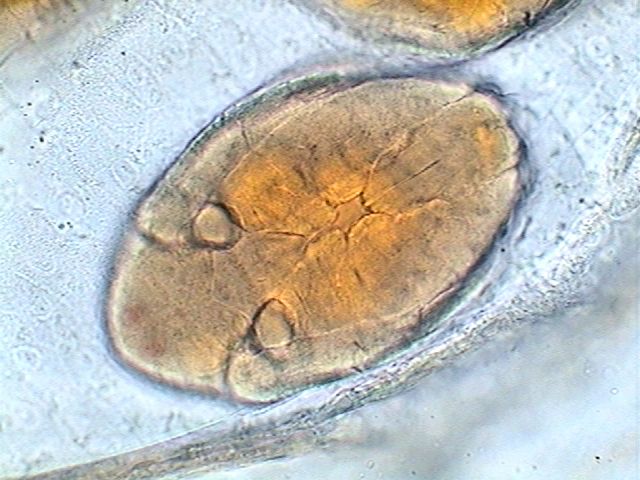
The brood pouch of a daphnia.
|
|
REFERENCES
Howard Webb -
MICSCAPE MAGAZINE,
November 2001
J.M. Cavanihac - Montage des lames,
MicrOscOpieS
M@gazine
Brian Adams -
MICSCAPE MAGAZINE, February
1999
Jean Legrand - Construction d'une hotte de
sechage,
MicrOscOpieS
M@gazine
Walter Dioni:
Micscape
Magazine.
SAFE MICROSCOPIC TECHNIQUES FOR AMATEURS. I - MOUNTING
MICROSCOPIC SUBJECTS:
| Part 1: Introduction - liquid media. December 2002 |
| Part 2: Soldifying media. January 2003 |
| Part 3 - Mixed mounting media - Part A; fructoglycerol and modified Brun's medium as mountants March 2003 |
| Part 3b. PVA-lactic acid and PVA-glycerol March 2003 |
| Part 3c - Gum arabic media March 2003 |
Microscopy
UK Front Page
Micscape
Magazine
Article
Library
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy
UK web
site at Microscopy-UK