
|
No
formalin, no mercury fixatives. (Part 2) |
|
|

|
No
formalin, no mercury fixatives. (Part 2) |
|
|
|
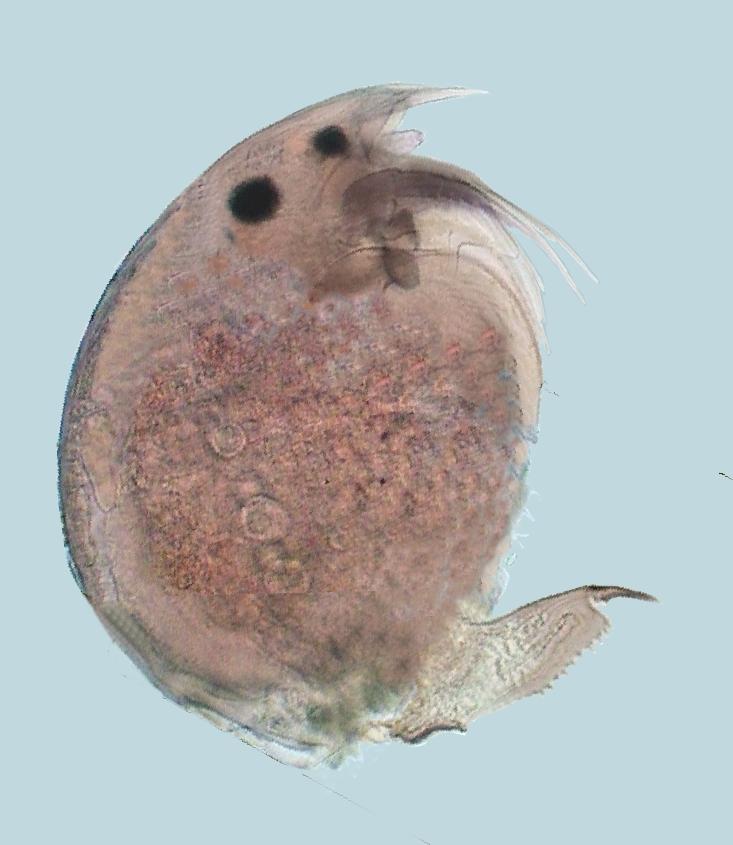
The title image is a
very young mayfly (Ephemeroptera) larva,
fixed in 70% alcohol and
mounted in PVA-Glycerol. Imaged with the 4x
planachromatic objective on a National Optical
microscope with a COL (circular oblique illumination)
filter. The field image width was 3.4 mm. This and all
the other images in the article were captured with the
digital camera integrated onto the
microscope.
|
I want to
discuss here three very well known fixing methods of quite
general use which can be complementary to the formulas
that I presented in the
first part. They
are fixatives for the use of biologists and microscopists,
rather than histologists. Although it is true that certain
fixatives used by the histologists can be very useful for
'microzoologists' and 'microbotanists', I think it is
better to present the histological fixatives without toxic
products in another article, if I have success in the
tests which I am currently making. (I speak of course of
non-commercial histological fixatives, because there are
several commercial products which are sold at very high
prices for the professional laboratories, and which are
well established.)
|
|
|
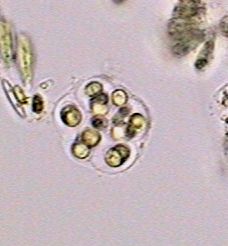
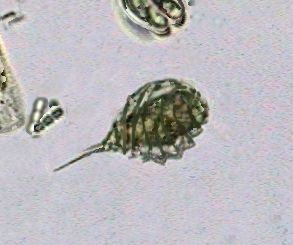
Chydoridae, fixed with 70% alcohol, wet mounted in
1:3 glycerin-water. Three image mosaic. Captured
with the x10 objective. Half reduced. Background
somewhat cropped. |
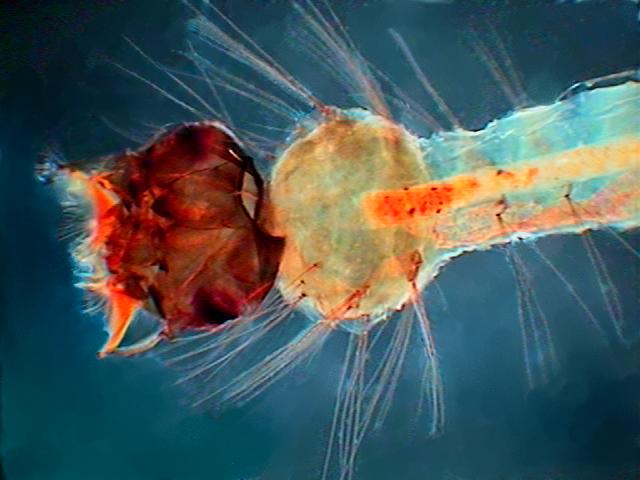
Young Culex mosquito larva. Fixed with 70% alcohol
many months ago. Mounted in PVA-Glycerol.
Captured with the 4x objective. Width of the field
of view 3.4 mm. COL filter. |
Alcohol 70%
is a standard fixative for many zoological groups, the
arthropods in particular. It behaves well with insects,
arachnids, crustaceans and larger animals. It is safe (if
you do not drink more than two glasses of wine per day!)
and the fixed samples are very durable. But it has a great
defect. It dehydrates specimens and contracts the tissues
a lot, and, employed alone, it is unusable with the
smaller microfauna which most of the time are
unrecognizable right after fixing. You can judge by the
included pictures that it is not the best fixative for the
smallest fauna.
If you want to have your sample fixed in 70% alcohol your
technique will vary according to the specimen. But the
general rule is to estimate the volume of the material
(plankton, sediments, algae, etc.) to be fixed, including
in the estimate the sample water volume. Then you add
sufficient alcohol at 90% or 96% to obtain the desired
percentage. The table below is a guide to make dilutions
at 70%. Do not be surprised by the figures. The table
takes account of the quantity of water in the added
alcohol. The Total volume is included as an aid for you to
place the sample to be fixed in a suitable bottle.
|
Sample
(ml) |
5
|
10
|
15
|
20 |
25 |
|
Alcohol
96 (ml) |
13.5 |
27 |
40 |
54 |
67 |
|
Total
volume (ml) |
18.5 |
37 |
55 |
74 |
92 |
|
Alcohol
90 (ml) |
17.5 |
35 |
52.5 |
70 |
87.5 |
|
Total
volume (ml) |
22.5 |
45 |
67.5 |
90 |
112.5 |
The drugstore
alcohol at 70% could be appropriate only for samples that
you can drain almost completely. Insects, small fish,
tadpoles, or even entomostraca collected with your net and
well drained. As the water of the sample will dilute your
alcohol, do not forget to do one or two changes with fresh
alcohol after 12 to 18 hours.
Alcohol is a "macroscopic" fixative. It does not fix the
nucleus well. To improve fixing, acetic acid is added. The
acid fixes very well the nucleus and moreover it expands a
little the tissues, counteracting the action of
alcohol.
Wolman has proposed a formulation to fix the sections made
with the freezing microtome:
Alcohol
96%..........................95 ml
Acetic
acid..............................5 ml
This formula is certainly of a more general use.
But, if you neither intend to undertake histology, nor
make taxonomic descriptions with very exact dimensions,
you can use only alcohol.
|
|
|
A chlorophycean algae, with a gelatinous envelope
of a species unknown to me. x40 objective.
Somewhat scaled up. Very cropped image |
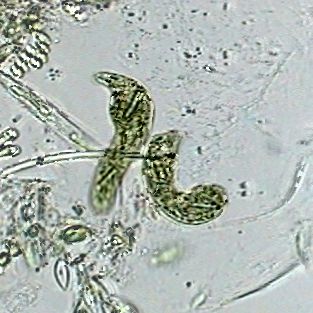
Two specimens of Euglena deses, objective
x40. 70% alcohol. Mounted in the same. Captured
inside the empty valves of a cladoceran. Cropped
image. |
Methyl alcohol (methanol) which once was cheap, was
formerly used mainly as a denaturing agent of "good"
ethanol, to prevent ingestion. Do not drink methyl
alcohol, it is fatal. But in many recent works it is
recognized that methyl is really as good as or better as a
fixative than the ethylic, and it is now proposed as a
total or partial substitution in traditional formulas.
One of them is Carnoy’s, which is today named Methacarn,
with its modified formula:
|
Methyl
alcohol...........60 ml |
Becuse of its fast penetration, Carnoy, and now
Methacarn, are used for chitinized organisms, and tissues
used in genetic investigations (vegetable roots, anthers,
buttons of flowers, testicles and ovaries, eggs of
insects, etc.). But also to fix smears of cytological
materials, or free living and parasitic protozoa. The
alcohols used in these formulas, both the ethyl as well as
the methyl are recommended to be absolute. Apart from
being very expensive, the absolute alcohols are really
almost impossible to be maintained in the amateur’s
laboratories. They absorb water from the air almost from
the moment at which the bottle is opened. 96% alcohol is
almost as good.
Actually some researchers reject the ethylic and use
exclusively the methylic (a little cheaper) or the
isopropyl alcohol (a little more expensive).
As they are interchangeable you can see that the
commercial alcohols denatured with another alcohol are
perfectly adequate for amateur microscopy. It is not the
same when the additive is a vegetable essence or another
oily substance which mixed with water becomes
opalescent.
I believe
that hot water (heat really) was recommended for the last
30 years of the former century (yes, the twentieth!), but
was only really accepted within the last 20 years of the
century. Most helminthologists usually employ it. It is
said that the treatment by hot water fixes the animals in
their normal aspect, in extension, without deformation,
and keep their true dimensions, which is very important
for good taxonomy.
|

|
|
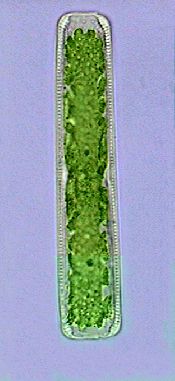
Diatom, the day after being fixed with hot water.
x40 |
Euglena deses. x100 0I. Hot water. |
|
Cropped pictures from wet mounts sealed with
nail polish. |
|
You can put your creatures on the slide in a drop of water
and heat from beneath with a match or a lighter. The
objective is to obtain a temperature in the neighborhood
of 50-60ºC. The tardigrades, the trematodes, the small
cestodes, nematodes, leeches, and others
micro-invertebrates respond very well to this operation.
With the larger helminthes you can even heat the fixative
and pour it on the worms in a dish.
For the smallest protists you put the sample, preferably
concentrated by filtration, in a container which accepts
four times the same volume. You wait until the animals
(rotifers, gastrotriches, nematodes, protozoa, etc.)
resume their normal activity, and you suddenly add a
double volume of almost boiling water. As Edmondson notes
(1959), organisms in the region where you poured the water
are certainly overheated, and the edge of the container
did not receive enough heat, but a ring located between
the two zones will present animals in a state of perfect
extension. Yes, you do have the task to seek them out drop
by drop, but Edmondson notes that it is the only method
which makes it possible to fix some specimen of
Notommata
(a very
difficult to fix rotifer) with its "auricles" spread out
well.
Generally after fixing by hot water, one makes a post
fixing with a conventional fixative (alcohol 70% for
entomostraca, lactocupric for the smallest invertebrates,
etc. according to the species present in the sample). I
always do this, and I advise you to do the same.
The professionals and some amateurs as well, fix some
materials, (primarily for histology), with the microwave
oven. This certainly requires a "laboratory oven" or the
careful calibration of your domestic one. It is possible
to do it. I calibrated mine.
|
|
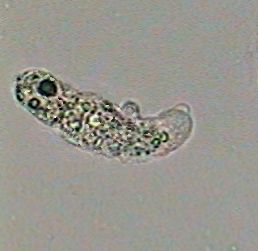
An amoeba at 100x killed by the boiling
water!! |
But the microwave times for the very small quantities of
the materials used are so short, and the adjustment so
difficult (because of the irregular distribution of
radiation in the space of most of the domestic ovens),
that I have given up after having cooked many nematodes,
rotifers and protozoa. The oven is better used to heat the
water or the fixative which you would use, or to dry the
border sealing your coverslips (for this last application
be very careful, start with a time of not more than 5
seconds of exposure at 100% power). If you want to heat a
larger sample ensure that the rise in temperature (even if
it is reached in 2 minutes) is gradual and not
instantaneous as when you add almost boiling water, the
surprise factor is lost and your subjects can
contract.
|
Iodine
crystals………………….. 5 g |
Keep in brown or opaque bottles. Add 0.5 ml to 100 ml of
sample.
All those who work with plankton samples know the
so-called "Lugol's solution" which is employed to fix such
samples. It is really Rhode’s fixative (Lugol's solution
almost 10 times concentrated + acid acetic, or potassium
acetate) which is employed. It is very suitable to
preserve phytoplankton, protozoa and rotifers in the
plankton, to which iodine (a heavy element) adds weight,
and cause their quick precipitation, allowing its
concentration.
|
|
|
|
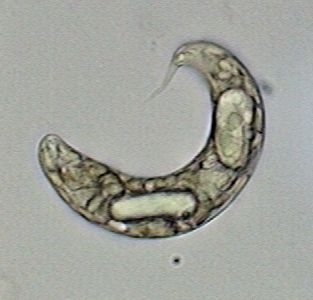
Euglena prob. oxyuris, x100 OI |
Collage of microflagellates x100 OI, taken from
several pictures. Click the
image to see a labeled copy |
Phacus pyrum, x100 OI |
The planktologists take their samples, put sub-samples in
special cylinders and let all the organisms precipitate to
the bottom. The cylinder bottoms have the thickness of a
coverslip and allow the observation and counting of the
organisms with an inverted microscope.
Don't you have an inverted microscope? Well, here is one
of the techniques which you can apply to study the very
interesting ultra-microscopic phyto and zooplankton that
pass through the usual plankton nets. Use a bottle or
other tall container of 1 or 2 lt. preferably a glass one,
and with a flat bottom or conical bottom. I have even used
inverted bottles of soda beverages, without its bottom.
Add 5 ml of Rhode's to the sample, mix it well and leave
in darkness for 2-3 days. With a flexible tube improvise a
siphon and discard supernatant water without agitating the
sediment, leaving at the bottom a tenth of the initial
volume. Now stir up, and pour the liquid in another
suitable tall and cylindrical flat or conical bottomed
bottle. After two days draw off most of the liquid, and
take drops of the sediment at the bottom to examine them
under your microscope.
You will be astonished by the great quantity of micro
phytoplankton species and even by the very small micro
zooplankton which you can now detect.
The samples fixed with Rhode’s can be preserved for a few
weeks, protected from the light. But the organisms take a
brown color which can obstruct the observation. To restore
to them their transparency add some small drops of a 5-8%
potassium hyposulfite solution.
For those who will carry out only some tests I give here a
formula which has given me good results, but which depends
on the quality of the medicinal tincture of iodine that
you can find in your pharmacy.
I start from a commercial solution of formula:
|
Iodine
crystals…………….……. 1.2 g |
I add 50 ml of white commercial vinegar (5% solution of
acetic acid)
My final formula is:
|
Iodine...............................1.2 g |
Which is more or less a Rhode’s diluted 4 times, by
taking account of the really active ingredients. For 100
ml of sample I add 2 to 4 ml of this alternative formula.
If you want to test this way study the formula of "your"
medicinal tincture of iodine and proceed in the same
way.
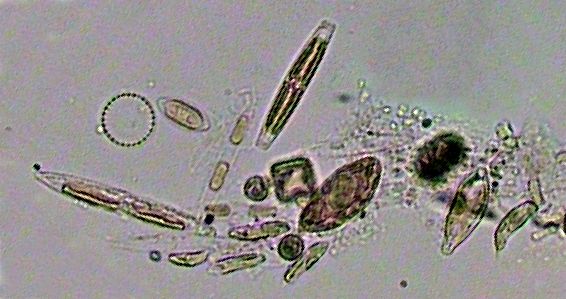
|
|
A miscellanea of decanted bacteria and
microphytoplakton. |
NOTE:
when you work with the other
fixatives, the cilia of ciliates and other invertebrates
are almost invisible. You can often highlight them by
adding a simple trace of Rhode's to your fixed wet mount.
Be careful: only one trace. This can highlight the cilia,
the nucleus and flagella also.
There are
other safe formulas and methods, that generally have more
specialized uses, but we have reviewed various
alternatives without any dangerous ingredients, which when
used with wisdom will allow you to work with an extensive
number of microalgae and microinvertebrates
species.
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line
monthly magazine of the Microscopy UK web
site at
Microscopy-UK