Inverted Microscopes
by David Goldstein, Seattle, Washington, USA
Inverted microscopes have some advantages and disadvantages
compared to a conventional microscope. However, if you can
overcome the major disadvantage (the cost of the thing), the
advantages are substantial for observing living microorganisms.
Introduction
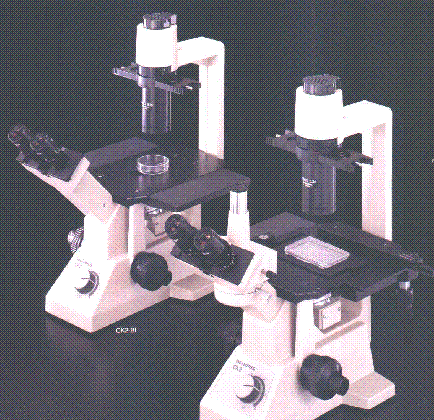 Recently, I became the
proud owner of an Olympus CK2 Inverted Microscope with the result
of some damage to my bank balance. However, I find myself using
it more and more often in preference to a conventional
microscope. The reasons for this are discussed in this article.
The Olympus CK2 with and without a trinocular head are
illustrated in the photograph to the right. I have the model to
the right with the trinocular tube.
Recently, I became the
proud owner of an Olympus CK2 Inverted Microscope with the result
of some damage to my bank balance. However, I find myself using
it more and more often in preference to a conventional
microscope. The reasons for this are discussed in this article.
The Olympus CK2 with and without a trinocular head are
illustrated in the photograph to the right. I have the model to
the right with the trinocular tube.
I should preface my comments by the limitations on my
knowledge. I am hardly an expert on the subject of inverted
microscopes. My entire experience with inverted microscopes is
limited to the several weeks I have spent with my CK2 (which I
understand Olympus is no longer making in preference to some
newer and more expensive CK models). I have not tried other
inverted microscopes although I have a brochure for Olympus' much
more expensive infinity corrected (IX) models.
What is an inverted microscope?
As the name suggests, an inverted microscope is upside down
compared to a conventional microscope. The light source and
condenser are on the top above the stage pointing down. The
objectives and turret are below the stage pointing up. The only
things that are "standard" are that (1) a specimen (as
dictated by the laws of gravity) is placed on top of the stage
and (2) thank heavens, the binocular or trinocular tube is not
upside down but in the standard position pointing at a
conventional viewing angle. As a result, one is looking up
through the bottom of whatever is holding the specimen and is
sitting on the stage rather than looking at the specimen from the
top, typically through a cover glass, as on a conventional
microscope.
The following illustration is taken from the
owner's manual and identifies the various components: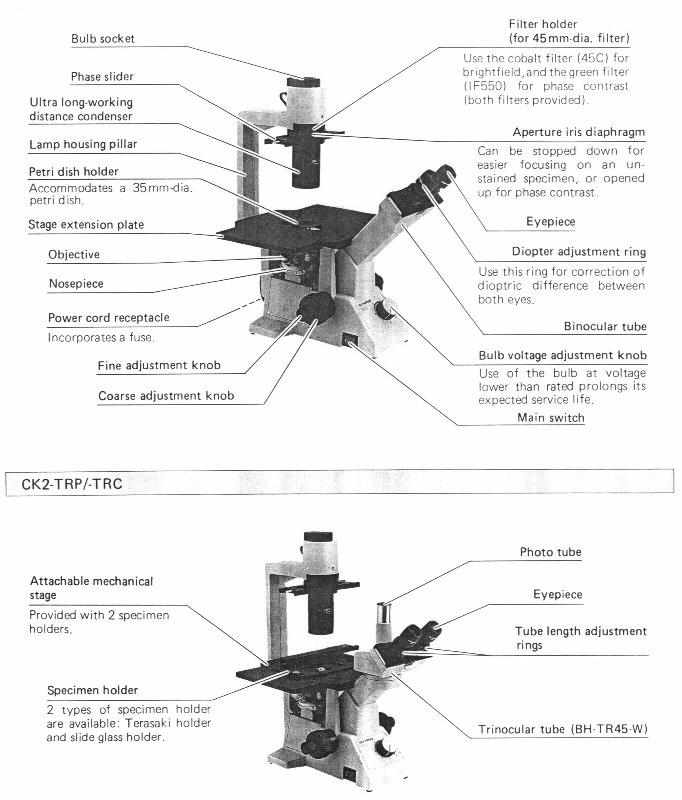
What are the Advantages of an Inverted Microscope?
Before getting to the advantage of an inverted over a
traditional light microscope, bear with me for a moment while I
discuss the advantage of a light microscope over an electron
microscope because the inverted microscope carries this advantage
one step further. The critical advantage of light microscopy over
electron microscopy is the ability to observe living organisms
and tissue. The various types of electron microscopes require
that the specimen be thoroughly prepared (which may include
coating it with gold) and placed in a vacuum chamber for
observation. Obviously, whatever life is in the specimen does not
survive this process. Light microscopes allow one to observe a
live microorganism such as a protozoan (now protist) as it goes
about its various life functions. While an electron microscope
has significantly greater magnification and resolution than a
light microscope (and some types can produce spectacular 3D
images), it only produces a snapshot in time of a dead subject.
As anyone who watches microorganisms for minutes and hours at
a time can testify, the moving picture that unfolds before the
observer of a live organism can reveal much about the organism
and its behavior that the momentary snapshot of a dead organism
will never show, no matter how detailed. Some organisms change
their shape so much from one second to the next that
photomicrographs taken seconds apart of a live organism may look
like they are of different organisms. It is for this reason among
others that light microscopy is well and alive as a research tool
despite the advantages of electron microscopy.
A traditional light microscope requires that the specimen be
placed on a glass slide, typically under a cover slip (although
there are objectives designed for use without a cover slip). This
usually means removing a small sample from the culture and
placing it in the artificial environment created by the slide and
cover slip. The temperature and oxygen content of the sample may
change quickly from that of the culture as a result. Further, the
organisms will be under increased pressure and in an unnaturally
confined space as a result of the cover slip. Also, the sample
will quickly dry out unless repeatedly replenished with water.
The loss of water by evaporation and the periodic adding of water
may change the salinity of the sample frequently. These changes
impose severe stress on microorganisms that can affect their
behavior and/or kill them in a short time.
Some partial solutions to these problems include making a
small chamber on the slide or using a slide with a well or
chamber built in and sealing it to prevent evaporation. While
this keeps the sample from drying out quickly, the inability of
the sample to exchange gasses with the air means that within a
day, a few days or a week, the organisms will die. Another
partial solution is to use the hanging drop technique in deep
well slide which prevents pressure and reduces confinement but
again is fairly temporary.
Therefore, observations through a standard microscope are also
limited in time. Its images are not the frozen image of an
electron microscope but neither can it easily allow study over
long duration (it is true that another technique available is to
use special reservoir slides with built-in water chambers that
allow the specimen to remain active for long periods but this
still requires specimen preparation and creates a relatively
limited environment for the life on the slide.
Would it not be nice is if you could observe microorganisms in
a large container under more natural conditions. This is what the
inverted microscope allows and by doing so, it extends the
advantage of the light microscope. Because of its configuration,
You can place an entire culture or large sample in a relatively
large container such as a petri dish and look at the entire
contents of the container under more natural (although still
admittedly artificial) and less stressed conditions. Such a
sample may sustain life over a much longer period. The cover of
the container slows evaporation greatly while at the same time
allowing some gas exchange. The larger quantity of water is less
subject to quick temperature changes although obviously, if
stored in a room, the water will acquire the room's temperature.
One sample of pond "scum" I have looked at over several
weeks still sustains life although the character of that life has
changed significantly over time from when I first collected the
sample. During this period, as the environment in the petri dish
changed, the life that it could support changed but there is
still a great deal of varied life.
Since inverted microscopes are often used for looking at
living organisms and tissue that may be killed by staining, they
often provide for "optical staining" through the use of
phase contrast or DIC. My CK2 has phase contrast. Rather than use
the more sophisticated phase contrast condenser used on a
standard microscope, there is a simple slider that goes through
the condenser and holds the necessary phase rings. Only the phase
rings for the lower power objectives (e.g. 10x and 20x) are
centerable by a fairly crude process (although it works). The
phase ring for the 40x objective is not but seems to work fine.
What are the Disadvantages of an Inverted Microscope
The first disadvantage is cost. Inverted microscopes are not
anywhere near as common as a microscope with a standard
configuration so there is less competition both in the new and
used markets. Further, they are more complex and therefore
expensive to build. One has to get an image that is pointing down
from underneath the stage up to the eyepieces in front of the
microscope and pointing up. One does not have to be an optical
engineer to see the complexities of this. Further, unlike a
standard microscope where focusing is done by simply moving the
stage or entire optical tube up and down, at least on my CK2,
only the turret assembly moves up and down. All of this adds
complexity and cost to the microscope.
On a standard microscope, higher power objectives are
optimized for a specific thickness of the cover glass which is
quite thin and uniform. With an inverted microscope, you may be
looking through the bottoms of different containers with various
thicknesses and variable optical characteristics. With higher
power objectives, it is advantageous to be able to correct for
the differences in thickness and on my 40x objective, there is a
correction collar which allows you to correct for container
thicknesses over a wide range (correction collars are also
available on 20x objectives although mine does not have one and
the images seem fine). However, again this adds to the complexity
and cost of the objective.
Also, remember that standard high power objectives typically
have a very short working distance and must get very close to the
subject to focus. This is why they are often protected by a
retracting nosepiece to avoid damaging the objective if it
accidently comes in contact with the cover slip and slide.
Because of the greater thickness of the bottom of the container
you are looking through (compared to a cover slip), a standard
higher power objective may not be able to get close enough to the
subject to focus. Therefore the higher power objectives on an
inverted microscope must be corrected for a much longer working
distance. These objectives are designated at least by Olympus as
"LWD" (long working distance) and "ULWD"
(ultra-long working distance) objectives. Again, this adds to the
cost. Further, even with all these corrections, they cannot make
up for the relative lack of optical clarity and uniformity of
looking through a good cover slip and therefore, the quality of
the image may not be as good as looking through a conventional
microscope with comparable objectives (note that plastic
disposable petri dishes are usually more suitable than most glass
petri dishes because they are thinner, more uniform and therefore
optically superior to the standard glass dish).
However, an inverted microscope is also excellent for viewing
pond life under a cover slip. The slide is inverted with the slip
facing the objective (the cover slip stays attached to the slide
despite the downward pull of gravity because of the surface
tension created by the drop of water). This eliminates the
compression problems experienced with upright stands. In
addition, protozoa and other invertebrates that normally move
along the substrate quickly move to the upper surface of the
slip, providing a much better optical link with the objective. In
fact, this is one reason the inverted microscope is such a hit
with cell biologists.
The condenser also must allow for an unusually long working
distance to allow larger containers to be placed on the stage and
on the CK2 is designated as ULWD. I assume as a result of the
necessary correction, it has an "N.A." (numerical
aperture) of only .3 and the 20 watt halogen light source which
is more than adequate on my standard microscope at high power is
just adequate with the 40x objective. This makes photomicrography
more difficult. I wish the CK2 had a 30 watt or higher light
source (as do Olympus' more expensive models). Surprisingly, I
have not noticed much difference in brightness or quality of the
image between viewing a petri dish with and without its cover
(even with condensation on the inside of the cover). As a result,
I tend to leave the cover on when I am viewing.
Another disadvantage is that the maximum magnification
available on an inverted microscope is more limited. Typically
40x is the highest powered objective (although Olympus has a 60x
objective available on its more expensive model). Oil immersion
100x objectives are often not available (although Olympus has one
for its IX series of infinity corrected inverted microscopes). I
do not know whether this is because of the difficulty of
maintaining an oil immersion drop upside down or the difficulty
of correcting such a higher power objective for long working
distances.
Finally, a mechanical stage is an extra-cost option on my
model and finding petri dishes or other containers that exactly
fit the holders that come with it are a little bit of chore.
However, one can become adept after some practice at moving the
container by hand without the mechanical stage even at high
power. This allows following a microorganism more easily through
a diagonal or zigzag course through the immensity of a 100 mm
diameter petri dish that may have a culture with a depth of
several millimeters.
However, whatever disadvantages an inverted microscope has,
these are far outweighed by the fundamental advantage of an
inverted microscope to accept a container with a large and
relatively long-lived diverse culture of live organisms without
any preparation.
A Possible Lower Cost Introduction to Inverted Microscopes
As prior Micscape articles on field microscopes have
noted, both the currently available Swift field microscope as
well as the no longer produced
Nikon Model H field
microscopes are forms of inverted microscopes. Nikon even offered
a special 100x oil immersion objective for its microscope. The McArthur microscope is
similar and may also still be available (click on "Nikon
Model H" and "McArthur to go the applicable Micscape
articles). The slide is placed above the objectives and one looks
up through the bottom of the slide. They may provide a less
expensive means of utilizing an inverted microscope if there is
enough room for a petri dish or other container on the small
stage between the stage and the light source (apparently the
Nikon does not have much space). Note that the Swift is by no
means inexpensive and I suspect the others, if available on the
used market are also in same category. Further, I have not had
experience with any of them.
Acknowledgments
I would like to thank Bill Amos and Ron Neumeyer for reviewing
a draft of this article and giving me their input which is
reflected throughout the final version. I would also like to
thank my wife, Sally-Jo for taking the time to read a draft of
this article and providing me with some helpful comments.
 Copyright 1998 David
Goldstein. Please click on my name to send me an e-mail.
I would appreciate any comments (including any errors you
notice). If you are curious, the following map shows where
Seattle, Washington is located in the United States:
Copyright 1998 David
Goldstein. Please click on my name to send me an e-mail.
I would appreciate any comments (including any errors you
notice). If you are curious, the following map shows where
Seattle, Washington is located in the United States:
© Microscopy UK or their
contributors.
First published in July 1998
Micscape Magazine.
Please report any Web problems
or offer general comments to the Micscape Editor,
via the contact on current Micscape Index.
Micscape is the on-line monthly
magazine of the Microscopy UK web
site at Microscopy-UK
WIDTH=1
© Onview.net Ltd, Microscopy-UK, and all contributors 1995 onwards. All rights
reserved. Main site is at www.microscopy-uk.org.uk with full mirror at www.microscopy-uk.net.
 Recently, I became the
proud owner of an Olympus CK2 Inverted Microscope with the result
of some damage to my bank balance. However, I find myself using
it more and more often in preference to a conventional
microscope. The reasons for this are discussed in this article.
The Olympus CK2 with and without a trinocular head are
illustrated in the photograph to the right. I have the model to
the right with the trinocular tube.
Recently, I became the
proud owner of an Olympus CK2 Inverted Microscope with the result
of some damage to my bank balance. However, I find myself using
it more and more often in preference to a conventional
microscope. The reasons for this are discussed in this article.
The Olympus CK2 with and without a trinocular head are
illustrated in the photograph to the right. I have the model to
the right with the trinocular tube. 
 Copyright 1998 David
Goldstein. Please click on my name to send me an e-mail.
I would appreciate any comments (including any errors you
notice). If you are curious, the following map shows where
Seattle, Washington is located in the United States:
Copyright 1998 David
Goldstein. Please click on my name to send me an e-mail.
I would appreciate any comments (including any errors you
notice). If you are curious, the following map shows where
Seattle, Washington is located in the United States: