Read other articles in the series.
Back to the medicine cabinet. If you have the occasional toothache or gum irritation (teenage girls loudly snapping bubble gum are particularly irritating), then you may well have a small bottle of Anbesol, Kanka, or some generic version of toothache relief fluid. These usually contain Benzocaine which is a topical anesthetic. I found one list of 45 different products which are used for toothache, gum irritation, or teething pains in babies. The solutions are flammable and so proper precautions need to be taken. As it turned out, I have both Anbesol and a generic version. Several years ago, I was experimenting with Anbesol, and mixing bits of other things, such as Ascorbic acid, with it and getting some very interesting and colorful results. Not too long ago, I bought a new bottle and when I tried it out, my results were not nearly as pleasing and I took a careful look at the liquid on the slide and noticed that it appeared rather oily or more viscous than that from the earlier bottle. I suspect that they have changed the formula and so altered the character of my crystals–talk about a lack of consideration; they didn’t even consult me.
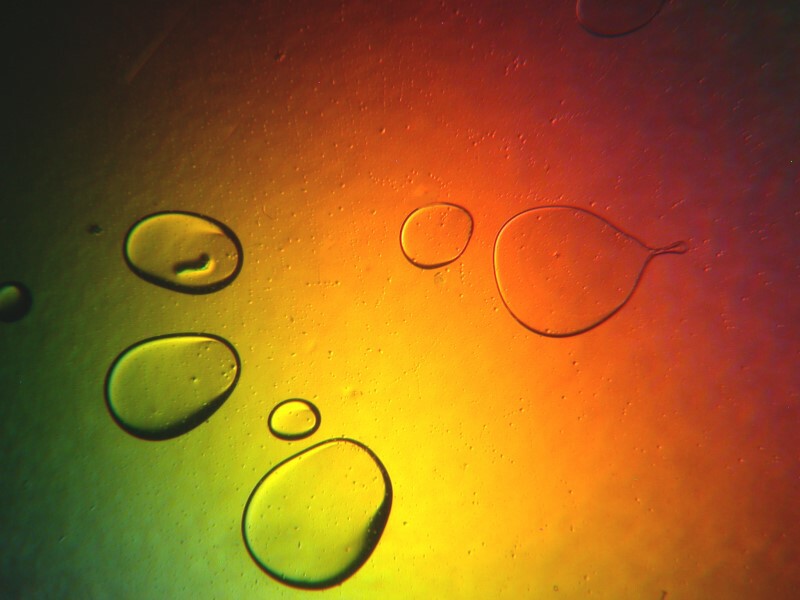
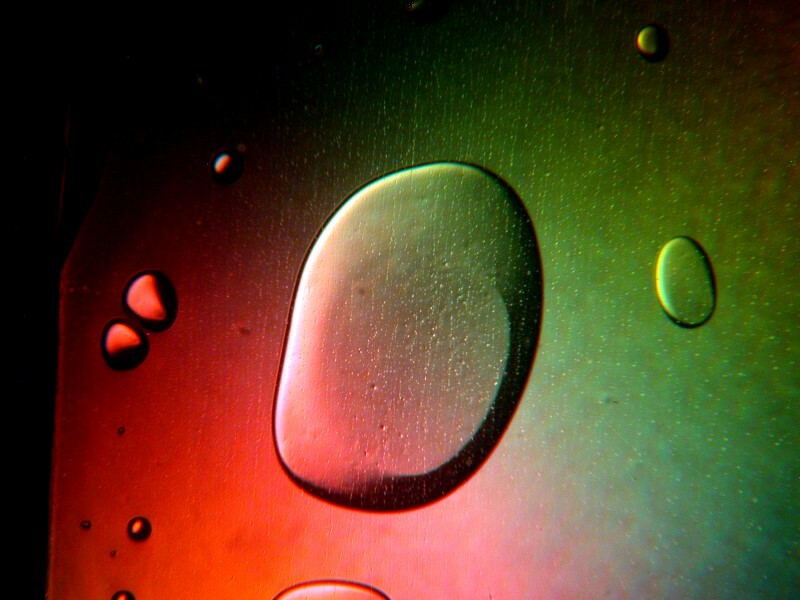
The older version of Anbesol also mixed very nicely with certain other sorts of things to produce some very pleasing results and I’ll show you a few examples in a minute but, first let’s look at 2 of just Anbesol itself.
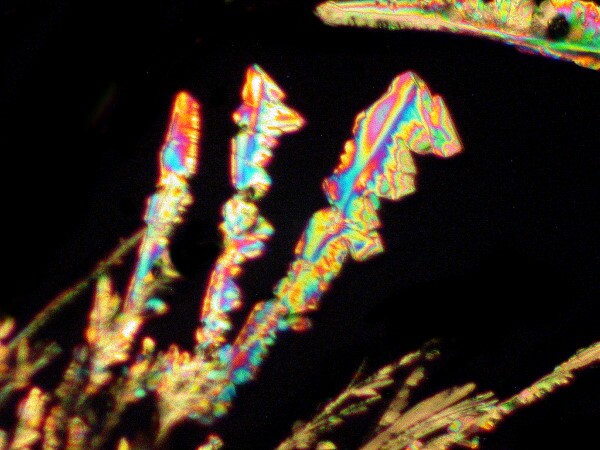
Then, I decided to get a bit adventurous and mixed a bit of Anbesol with a drop of Boric acid and a drop of Stevia extract. Stevia is a plant and an extract from its leaves has been used for over a thousand years in South America as a natural sweetener. This combination produced both colorful images and interesting forms. I’ll show 4 examples here.

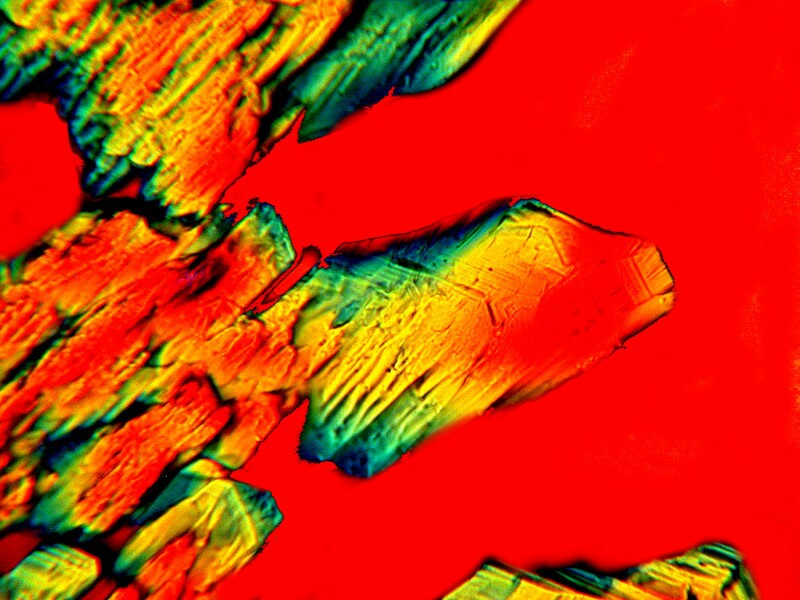
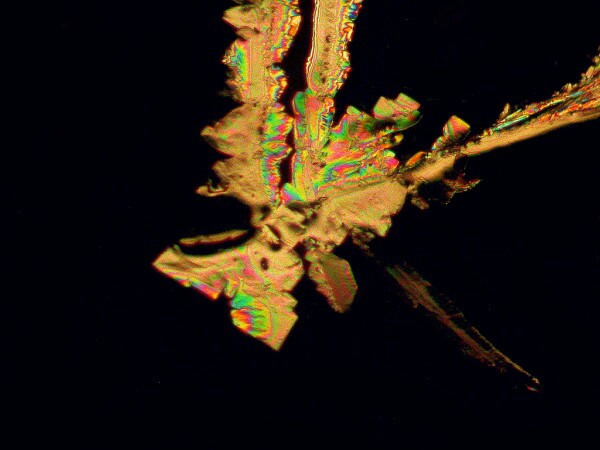
Next is an image of just Anbesol and Stevia. Here we get feathery crystals.

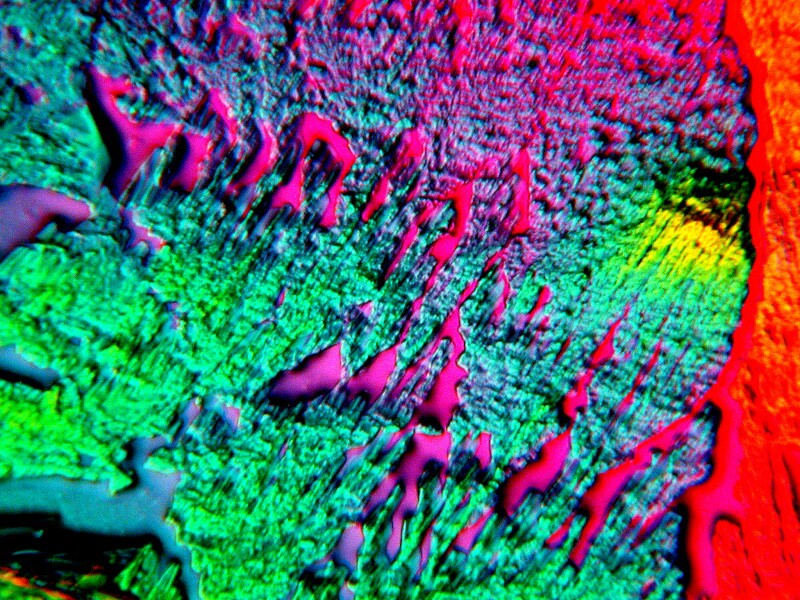
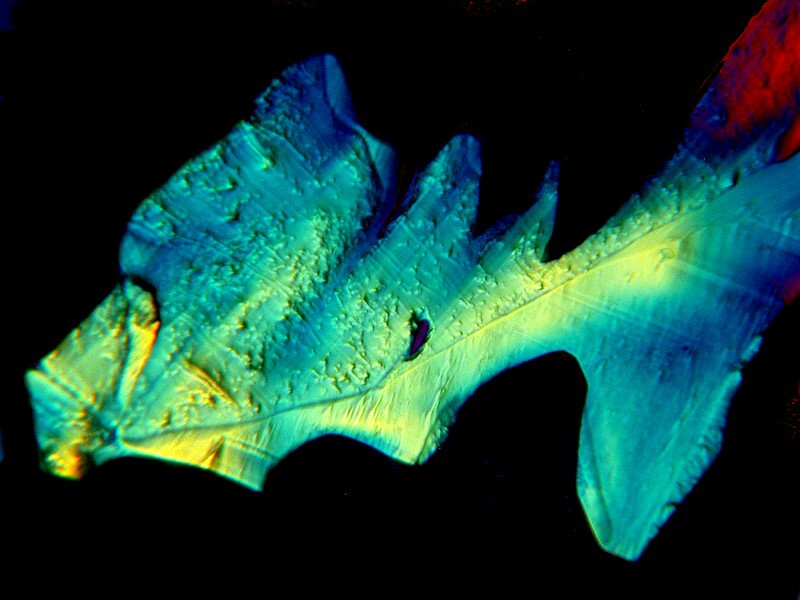
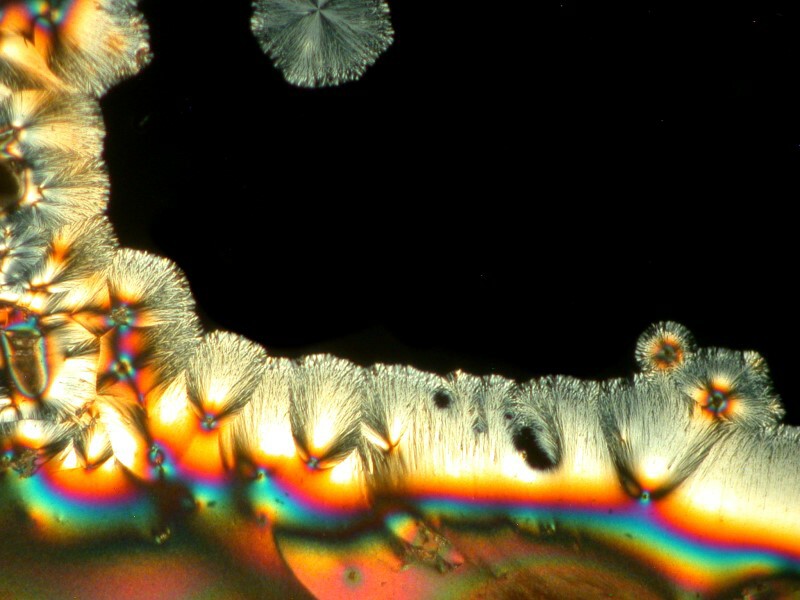
The generic toothache medication is very interesting on its own and produces intricate interlacings of crystals as you can see in the 2 examples below.


The generic form also produces some very unusual forms when mixed with other substances, particularly Ascorbic acid. Always keep some on hand and make up fresh solutions as needed. If you don’t have the chemical version, just go to the drugstore and buy a small bottle of Vitamin C and then grind some up and make your own solutions.
In this first image, along the lower portion we can see a row of fused disk-like shapes with mutant Maltese crosses in them and above, a portion of a rather typical disk formed by Ascorbic acid.
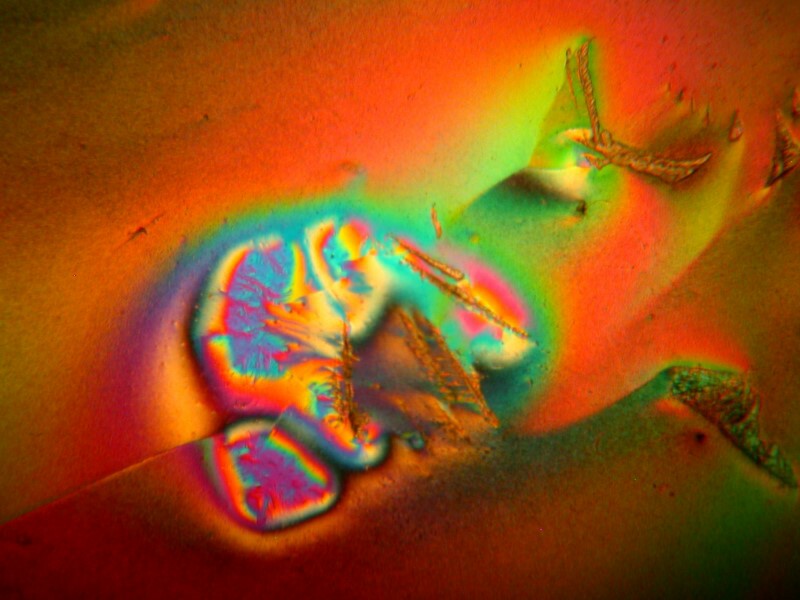
However, on other portions of the slide, the images become progressively more amorphous and less typically crystalline.
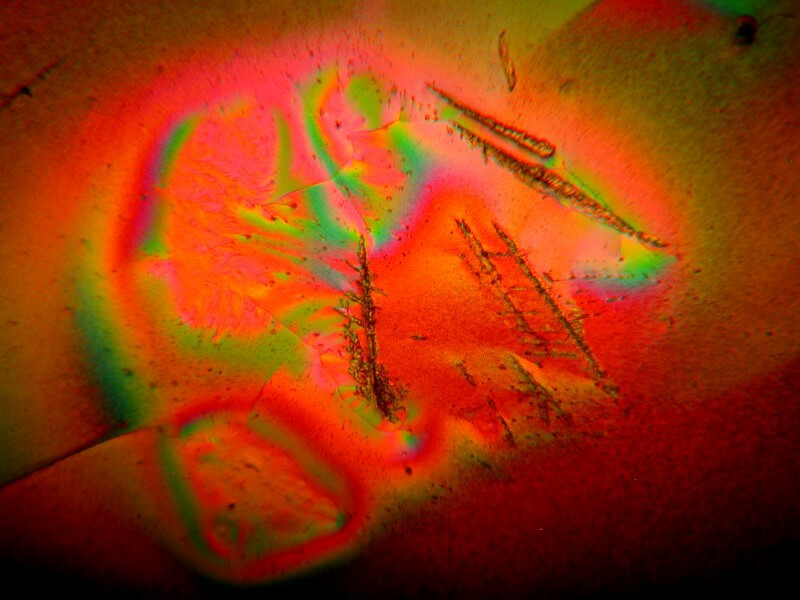
So, clearly, not all toothache meds are created equal and a bit of careful experimentation (they are, after all, flammable) can yield some fascinating results.
One of the rewarding aspects of investigating household products is that one sometime gets surprises from items which one may approach with considerable skepticism as good possibilities.
Who, for example, would think of Elmer’s scrapbooking glue as a likely candidate? I tried it on a whim and got the interesting result shown in the 2 pictures below. Now, I am considering mixing it with some other things to see what kinds of results emerge.
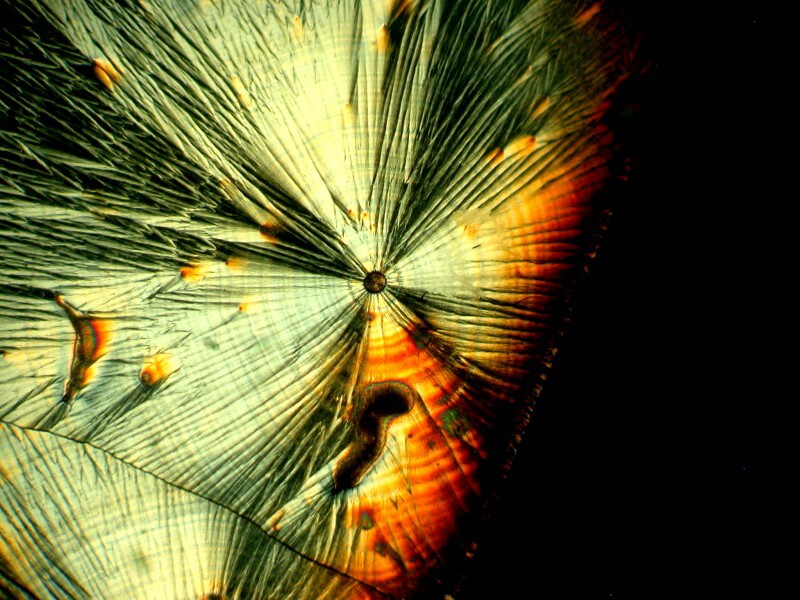
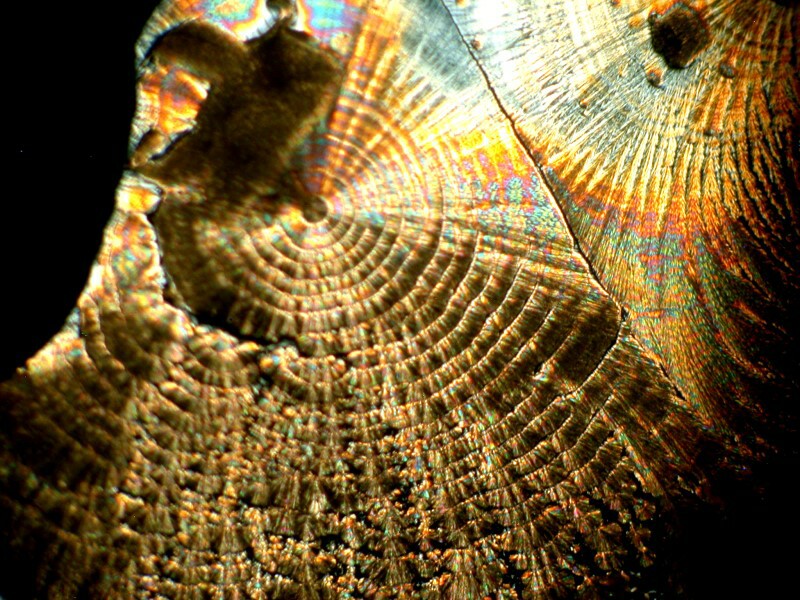
While we’re talking about glues and resins, some years ago, I chanced across the fact that certain types of mountants are birefringent. Recently, in one of my cabinets, I came across a small bottle of synthetic mountant from the old firm Turtox and this is their CMC9AB. I mixed a bit of Ascorbic acid with it and got some quite nice results. I’ll show you 2 examples.
If you have some old slides (or some sloppy ones like I make), look along the edges where the mountant has oozed out and dried. Use polarized light and you may be pleasantly surprised.
While I was indulging my eccentric whim, I took a small sample of a floor coating resin that my wife used to use and again mixed a bit of Ascorbic acid with it and here is the result.
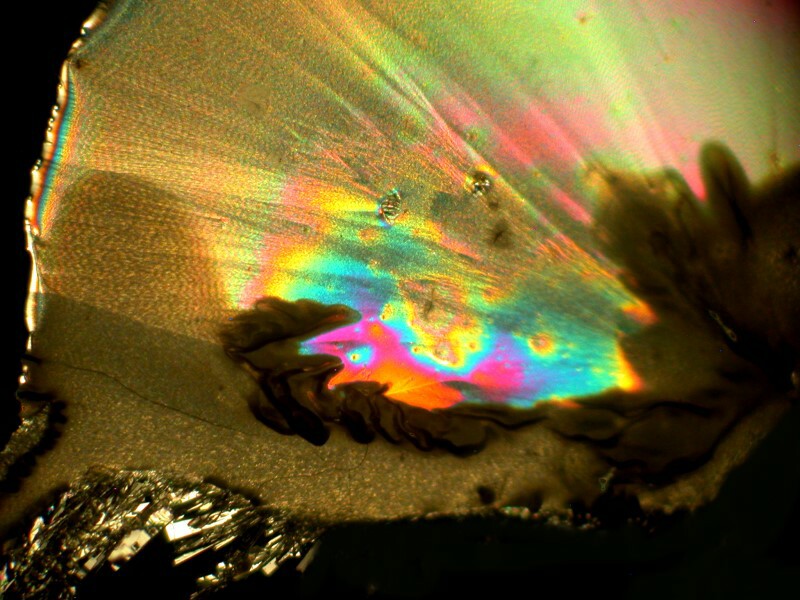
Emboldened by my enjoyment, I decided to go even farther out with my experimentation. Back at the medicine chest, I found some capsules of Simethicone which is used for the prevention of excess intestinal gas. Naturally, a bit of Ascorbic acid was added and I got a cosmic horsehead with an elongated neck, the horizon of a planet with 2 moons, and a space butterfly all of which you can see below.
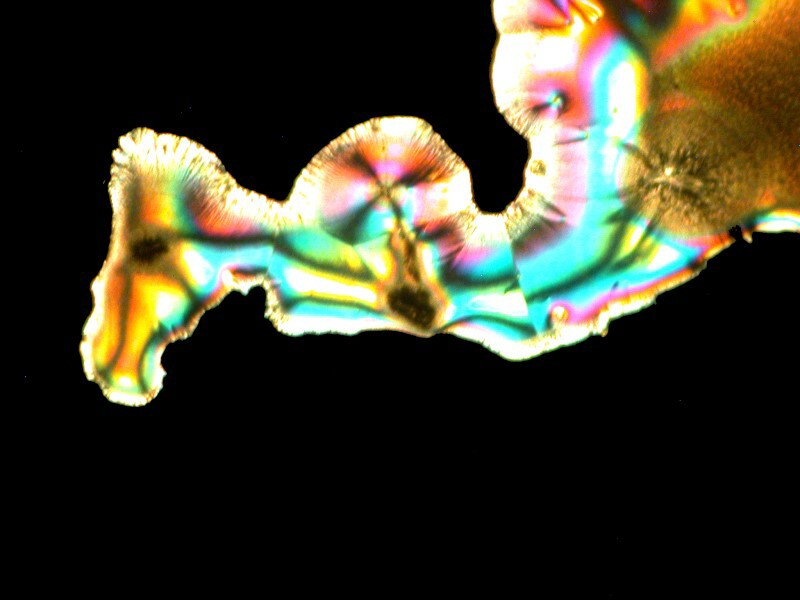
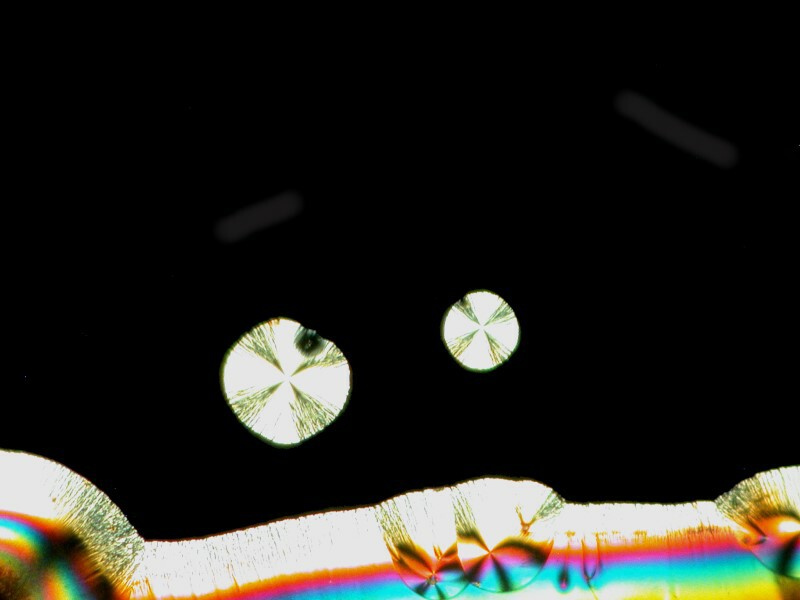
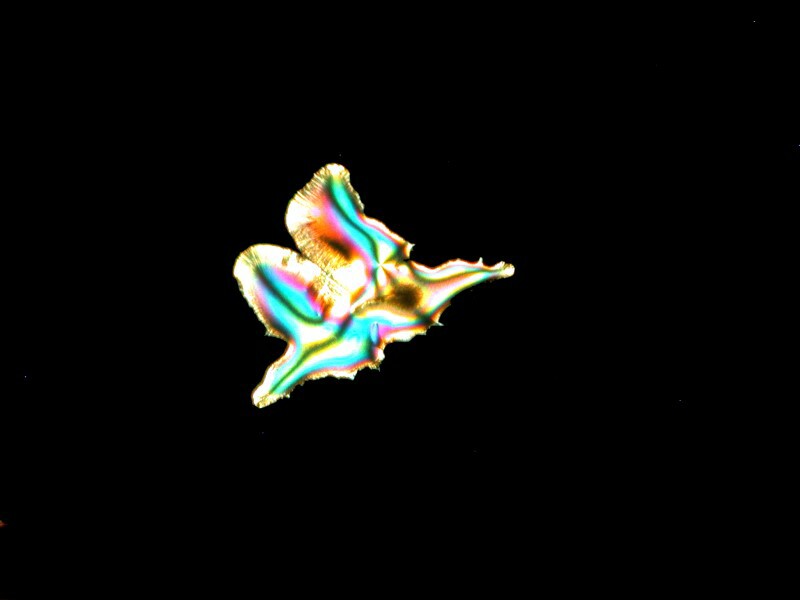
Crystals can be more fun than a barrel full of monkeys. What a silly language English is; imagine a large, round container full of smelly primates–-how could anyone think that would be fun?
However, while we’re on stomach and intestinal phenomena, I wondered what would happen if I made a preparation of proton pump inhibitor (which sounds like some part of a nuclear reactor), so some scrapings from a Prilosec tablet were placed into a solution of Ascorbic acid. This produced a planetary horizon being bombarded by a series of disk-shaped asteroids complete with a modified Maltese cross in the center of most of them.

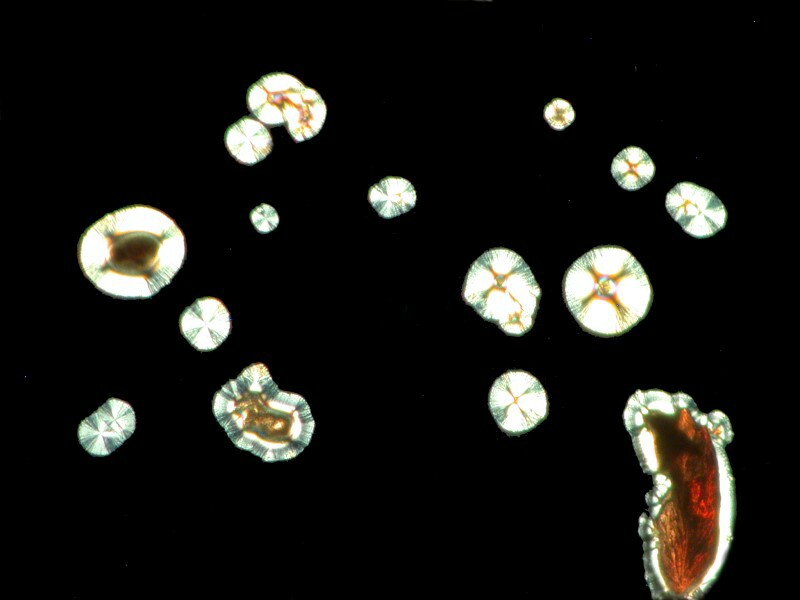
While we’re delving into the nether regions, I took a capsule of Docusate sodium which is also known as “stool softener” (take note, however, it doesn’t work on chairs, benches, or settees). It is, of course, a medication to soften up fecal material and ease its passage out of the body. My preparation included Ascorbic acid of course and the results were interesting in 2 ways. 1) the mixture produced webbed dome like structures and closeups of the webbing shows intricate patterns and 2) a slight shift in the lighting produces a surprising change in the images.
First, the web patterns:
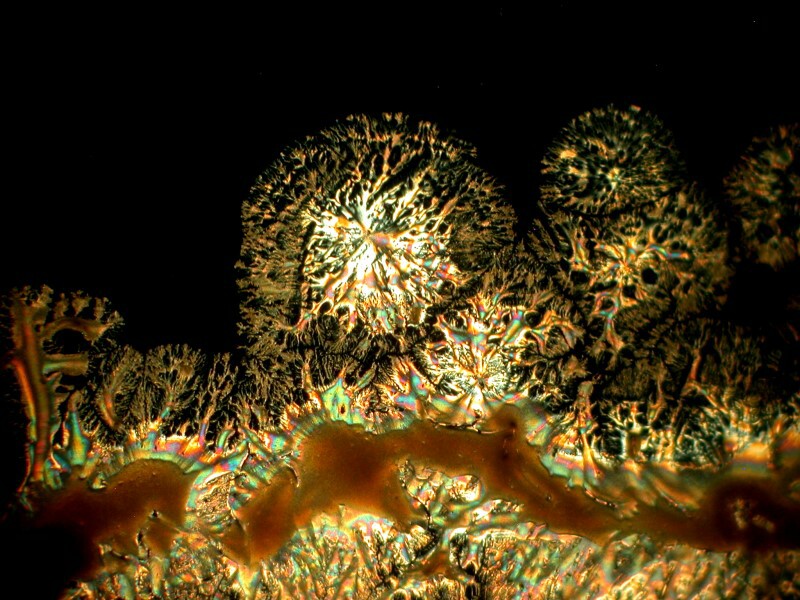
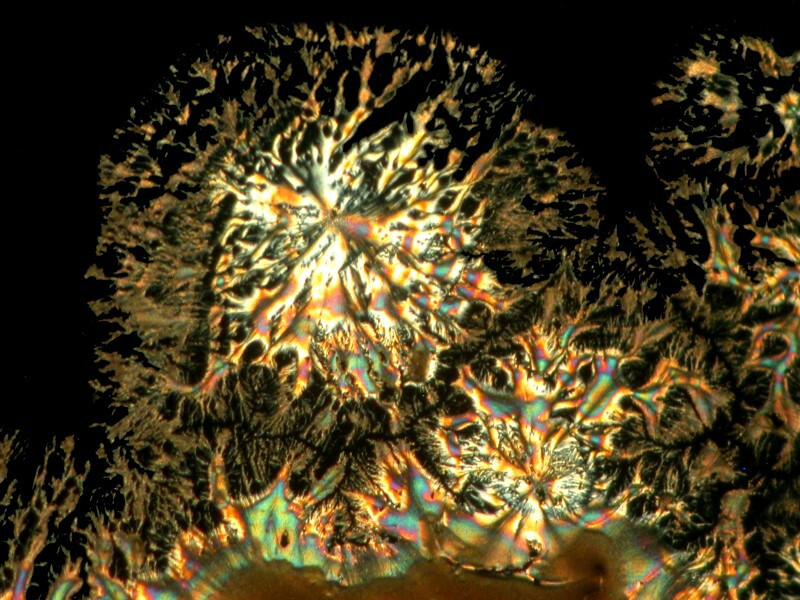
Next, a closeup.
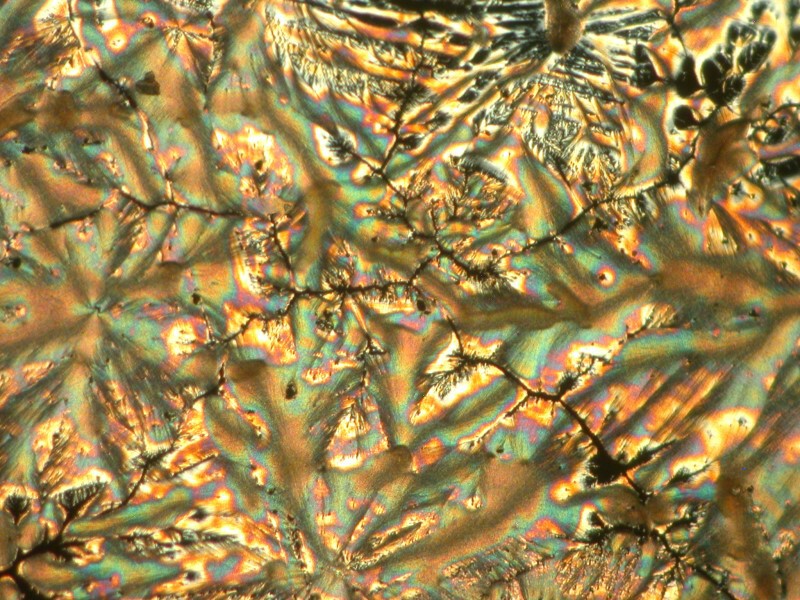
A slight shift in the lighting by changing the angle of the substage mirror.

Another closeup.
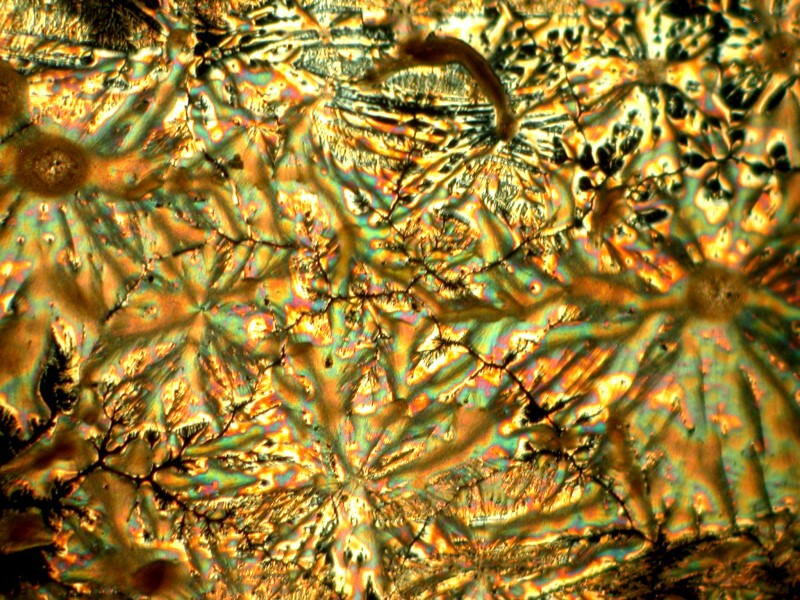
Another shift of the mirror.

Well, after all these anatomical adventures, you may feel like you need to clean off your hands. For that we can use some Purell, which is a hand sanitizer which is 70% Isopropyl alcohol. In addition, water, Caprylyl Glycol, Glycerin, Isopropyl Myristate, Tocopheryl Acetate, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aminomethyl Propanol, Fragrance. It should be noticed that this is the “Advanced” version. I guess earlier there was a “Backward” version. After reading the label, I thought to myself that I needed to mix some Ascorbic acid with it and certainly with all of those esoteric ingredients something interesting should happen. And indeed it did. I’ll show you 4 images which have, in my view, a remarkable variety.
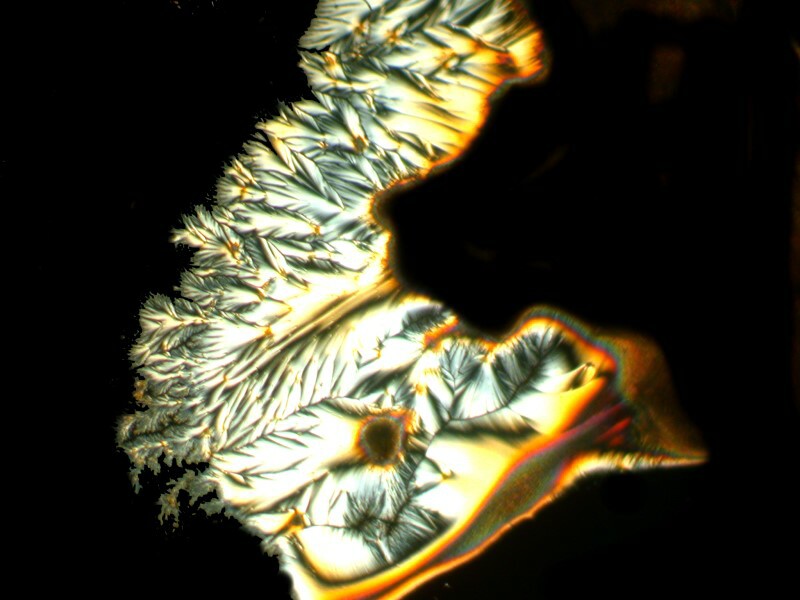
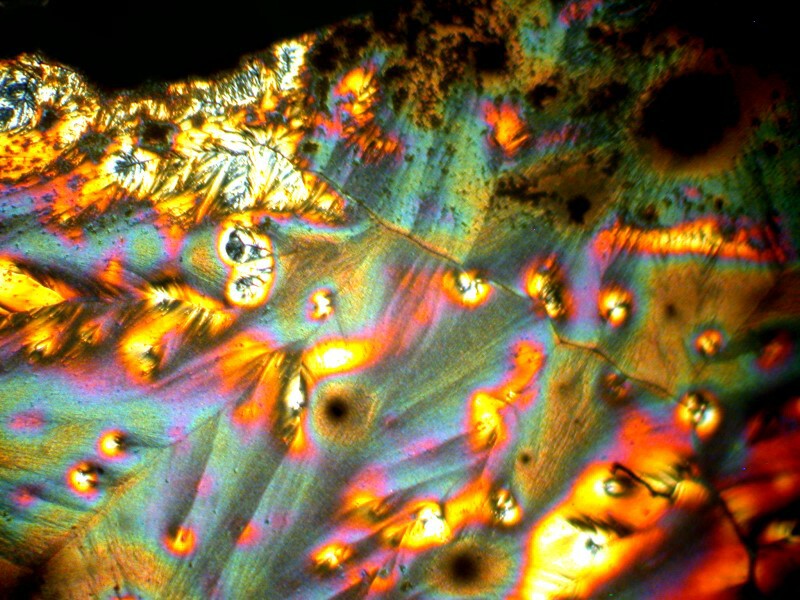
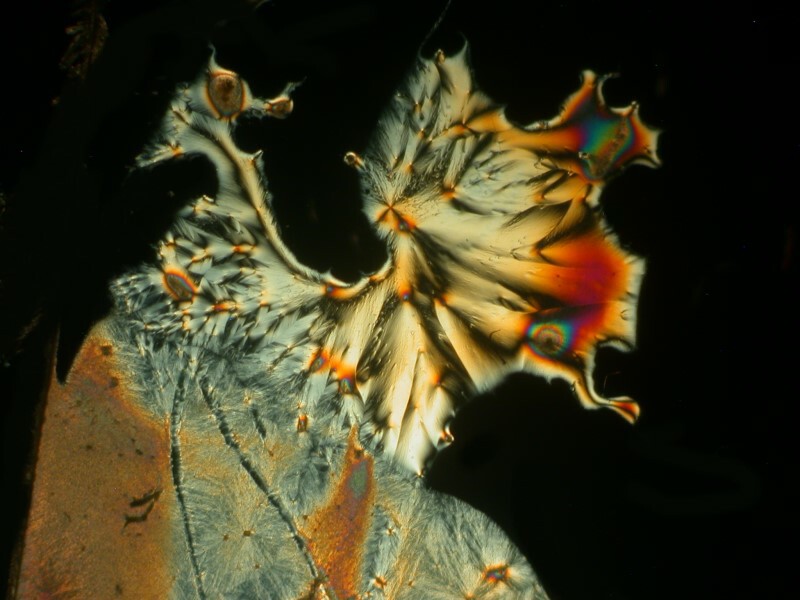
And my favorite.
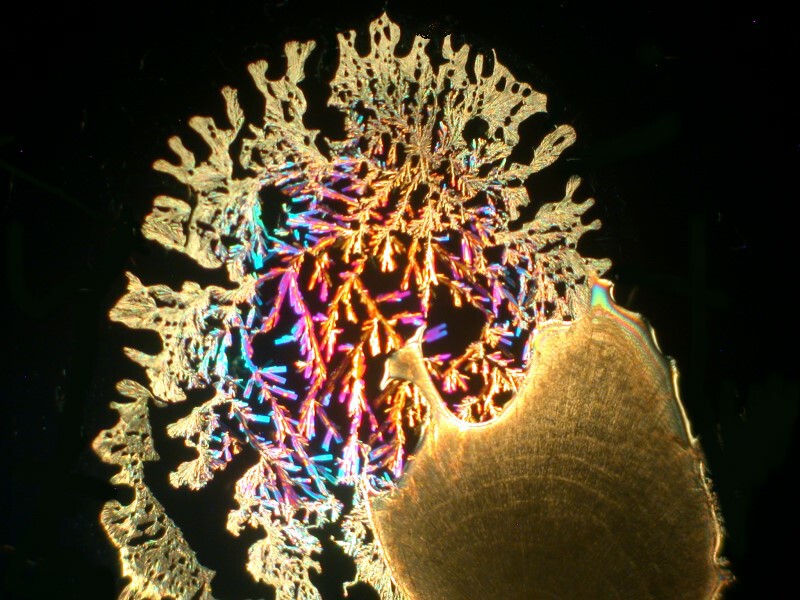
Who would have thought that hand sanitizer and a little Vitamin C could produce such remarkable micro-crystalline universes?
In Part 4, we’ll look at some more household products and a couple of chemicals which while they are not something one ordinarily finds around the house, are nonetheless, fairly readily obtainable.
All comments to the author Richard Howey are welcomed.
Editor's note: Visit Richard Howey's new website at http://rhowey.googlepages.com/home where he plans to share aspects of his wide interests.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
© Microscopy UK or their contributors.
Published in the January 2016 edition of Micscape Magazine.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk .