Read other articles in the series.
In Part 1, we looked at Bromo-Seltzer; in this part I want to start by looking at the company that produced Alka-Seltzer much later. However, first we need to consider its earlier product that earned Dr. Franklin Miles a fortune. In the 1884 Miles Laboratories began marketing Dr. Miles Restorative Nervine and guess what one of its main ingredients was–you got it, bromides! There were also other tonics, liver and blood cleansers, all promoted through extensive advertising using not only newspapers, brochures, calendars, and almanacs, even little books with health tips for householders. In the early 1890s, the advertising budget exceeded $100,000 which in today’s money is about 2 and ½ million dollars!
As was typically the case, the advertising was designed to convince would-be customers that it was a benefit for whatever aliment they might suffer from and that Nervine promoted general health. Clearly, from the name, the focus was on nerve-related disorders but it was touted as aiding heart problems, dealing with advanced age, various types of social stresses and the bad effects of smoking and stimulants. In addition, one of the calendars informs us that not only is it good for nervousness, but sleeplessness, headache, epilepsy, fits, shortness of breath, palpitations, and pains in the chest as well. Other advertisements enlighten us further by telling us the “Nervous Women are the First to Lose their Youth and Charm” and that “NERVES Hasten the Coming of CROWS FEET”. It even claimed to produce such calm that one would no longer be bothered by obstreperous children. Oh, where oh where is this magic elixir when we need it in this high-tech world?
However, Miles Laboratories didn’t rest on its laurels; in 1931 they introduced Alka-Seltzer. This was directed toward relieving cold and flu symptoms such as fever and indigestion, but was ostensibly good for hangovers as well. This product, however, was trying to remain simple and provide a “pleasant drink” without side effects. So, you can probably guess what it was initially composed of–aspirin, citric acid, and sodium bicarbonate (for the fizz, of course–“Plop, plop, fizz, fizz / Oh what a relief it is.” Today it comes in 17 different varieties of packaging from gel caps to liquids to tablets; however, in these products aspirin has largely been replaced by acetaminophen. Clearly, it has been an enormously successful product and it is still popular 84 years later. In 1978, Bayer AG, the giant German pharmaceutical company bought Miles Laboratories for $253 million which in today’s dollars is about $906 million but, of course, with all the new varieties, it would be worth much more now.
I know that I showed you Acetaminophen, Citric acid, and Sodium bicarbonate images in Part 1 but, as you surely guessed, I still have some and I’ll be glad to share them with you here along with a few images of Aspirin which we didn’t look at last time.
First, let’s see what happens when we place a drop of Alka-Seltzer solution on a slide. In the first 2 images, we get oddly amorphous shapes which I suspect is in part a consequence of using a cover glass.

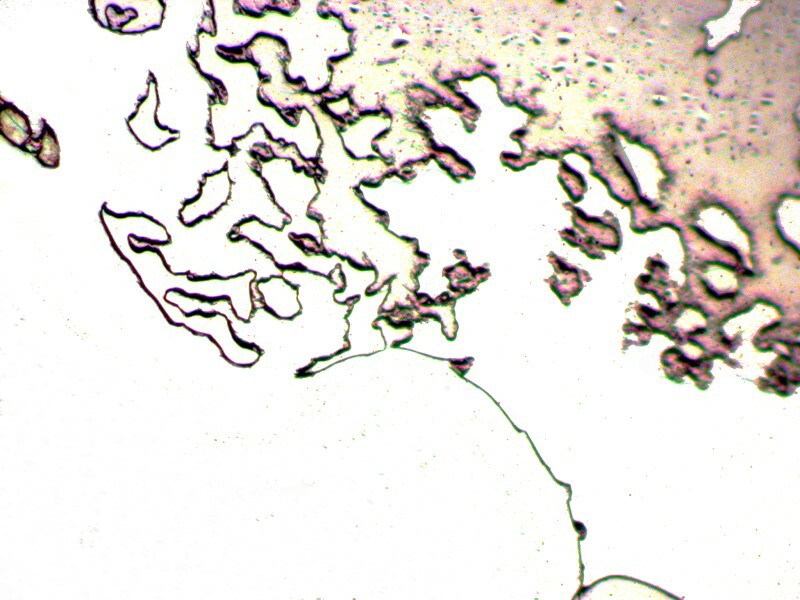
On the second slide, I just placed a drop on the slide and let it dry without covering it. It is not surprising that it produced a crusty blob, but one which under polarized light is quite colorful–first a brightfield image and then a polarized one.
Next a thicket of needle-like crystals of Sodium bicarbonate first using dark background and then oblique illumination.


And once again, Citric acid which is almost always colorful and morphologically interesting; the first image uses both oblique and polarized illumination and the second is simply polarized.
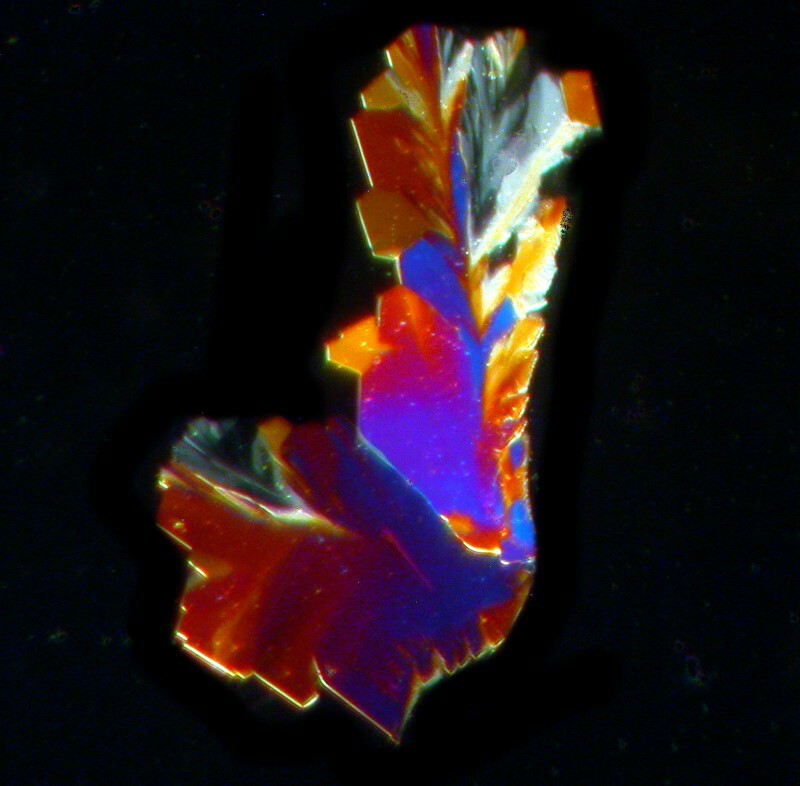
Now, we come to Aspirin. Most of the clustered crystallization tends to take place around the edges of a drop as you can see in the image below.
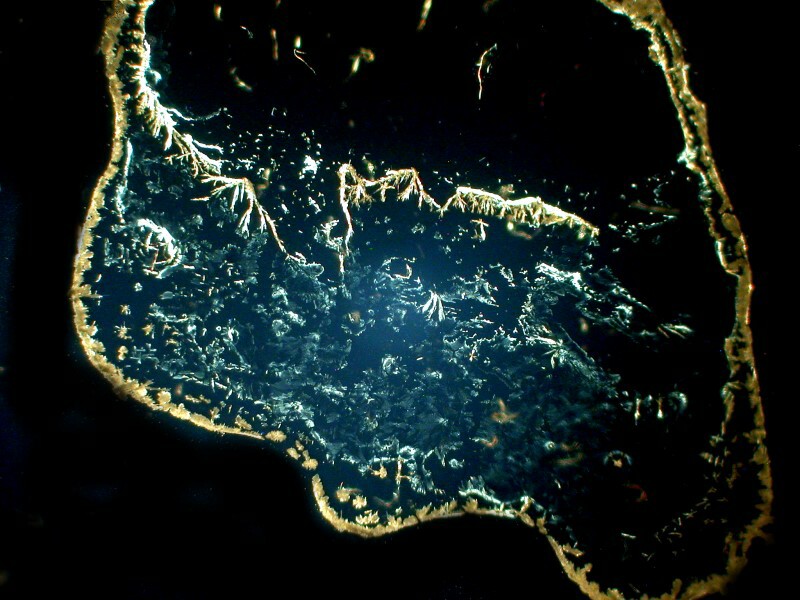
However, the clusters of small crystals are highly branched as is evident in the next 2 images. The first is brightfield and the second is polarized.

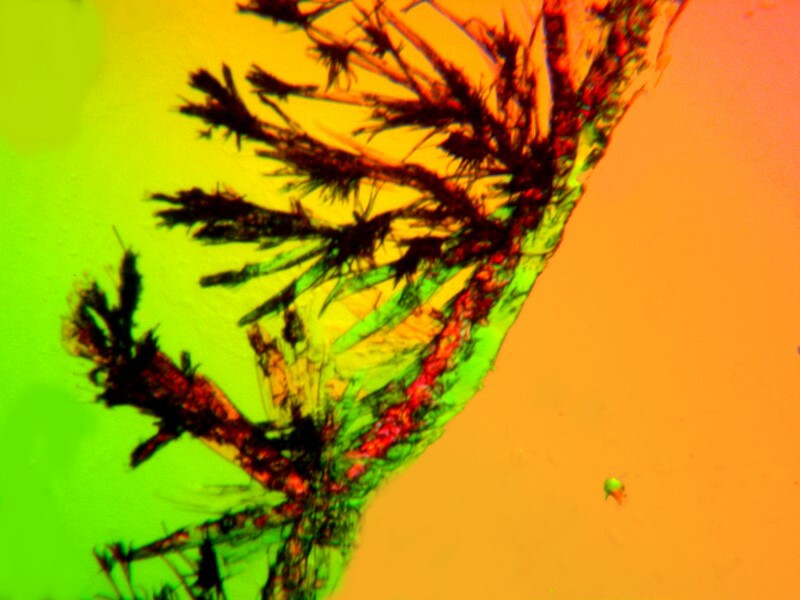
Then with Acetaminophen we once again see the crusty blob, but in this image we can also see a large number of tiny granular crystals.
Before we proceed to some more modern materials likely to be found in our medicine chests, I would like to take a short side trip into the past when patent medicines were at their peak (last half of the 19th Century and first half of the 20th Century) and take a look at some of the astonishing substances that were readily available. Most people know that Coca-Cola started out as “coca wine” and thus had both alcohol and cocaine in it and was recommended to relieve exhaustion. There were also Cocaine toothache drops; Bayer marketed Aspirin and Heroin as a cough suppressant; Amphetamines were used for weight loss; compounds containing Methamphetamines were claimed to be effective for relieving mental depression; Butabarbital was recommended for coping with menopause; for pain relief, you could purchase Pantopon Roche injectable Opium; for cold and cough Ayer’s Cherry Pectoral which came in two varieties, one containing morphine, the other heroin. However, I think my two favorites are Dr. Batty’s Asthma Cigarettes which are “Not recommended for children under 6" and Dr. Ham’s Aromatic Invigorator. This was one of many cure-all products for which no ingredients are listed and, on its label, it claimed to be a cure for “Dyspepsia, Low spirits, Nervousness, Heartburn, Colic Pains, Wind in the Stomach or Pains in the Bowels, Headache, Drowsiness, Kidney and Liver Complaints, Melancholy, Delirium Tremens, and Intemperance”. Something every medicine cabinet should have. And finally, we mustn’t forget the tonic for the geriatric set, Geritol, which claimed to have twice as much iron as a pound of calf’s liver. It also had some B vitamins and 12% alcohol in its early formulations.
I’m afraid my medicine cabinet is lacking in anything so exotic, but sometimes for chest congestion I take a swig of a syrup called Guaifenesin which my doctor assures me is both relatively harmless and beneficial, but it has that ghastly faux-cherry taste that pharmaceutical manufacturers use to nauseate their customers. However, mixed with a drop of Ascorbic acid, it gives some very interesting microscopic results.
These 4 images are all with polarized light and the first one shows a definite dominance of Ascorbic acid with the large central disk.

The other 3 show the definite influence of the Guaifenesin and, the next 2, I find quite elegant. The third one is a closeup showing the way in which those crystals formed.
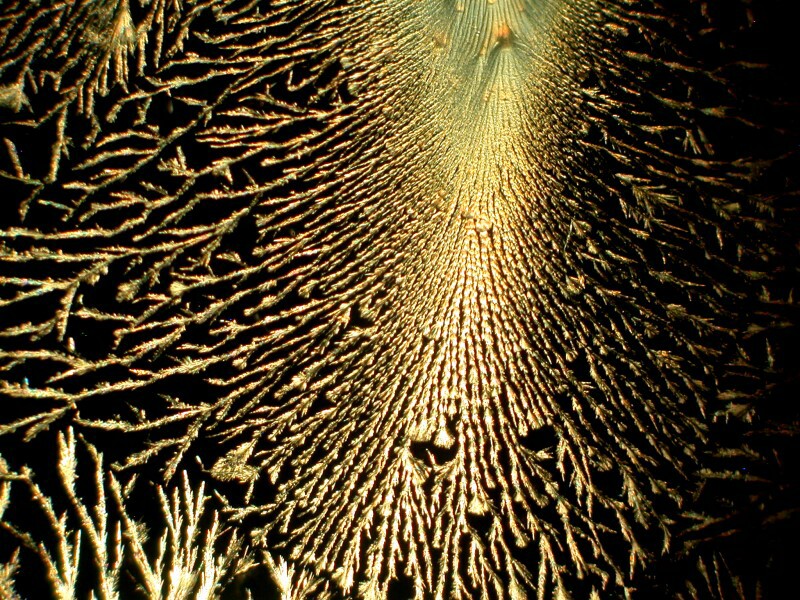
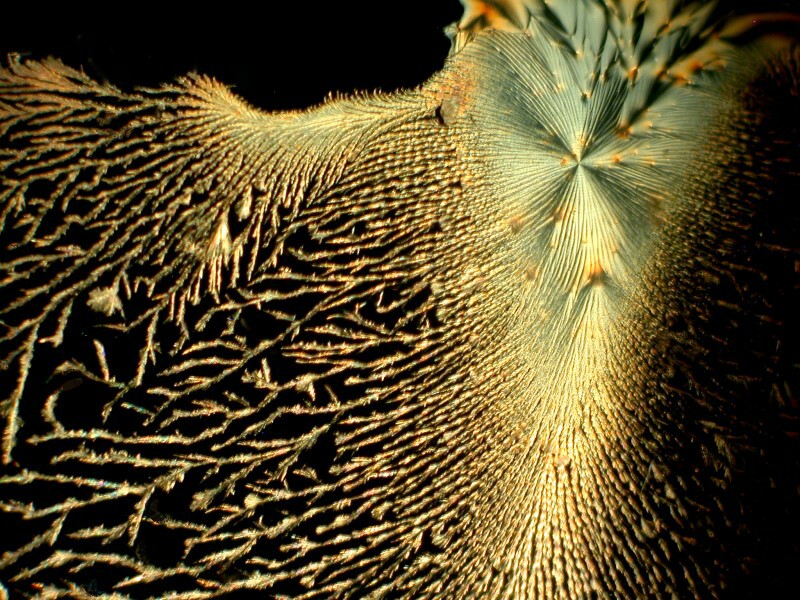

While rummaging around in my cabinets, I found an old bottle of Mercurochrome which if you’re under 35 you probably never heard of. For a very long time, it was the skin antiseptic of choice among grandmothers, mothers, and even children because it didn’t sting like iodine and it gave your injury a distinctive neon pink color. It was a 2% solution of Merbromin, an organomercuric salt and fluorescein. It was banned in 1998 in the U.S. and in the early 21st Century in France and Germany, but remains available in most of the rest of the world and is widely used because it is very inexpensive. Organic mercuric compounds are potentially highly toxic and although there is little research to suggest that Mercurochrome posed a real threat, the FDA removed it from the GRAS (Generally Regarded As Safe) list. There are now look-alike substitutes and some even still use the old Mercurochrome name, but they consist primarily of Benzalkonium chloride and Lidocaine. On AMAZON.COM, there are people complaining that they can’t still get the real stuff, but one person helpfully provides an address in Australia where it is still available. I know that my little bottle is the genuine stuff because it states on the label that it is a “2% Aqueous Merbromin Solution.” I don’t use it for anything but making combinations of crystal solutions, since I do take the toxicity of mercury quite seriously; when I want some mercury, I have a nice big piece of swordfish steak.
The following 3 images are a mixture of Mercurochrome (the genuine stuff) and Ascorbic acid. The third image is a closeup of the disk in the first image.

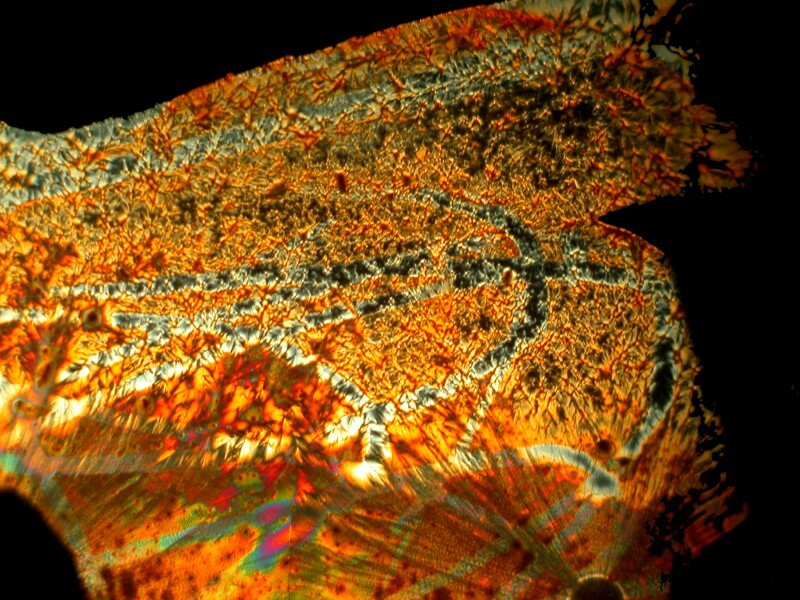

Some of you may think it’s unfair that I keep using substances that are no longer available in the average household, but I warned you early on that we have an eccentric household. However, I’ll try to make it up to you here and in the next essays. To finish this one up, I’ll look at two brands of widely available eye drops. I told you at the end of Part 1 that I would raise the issue of active and inactive ingredients again and so here we go.
1) Visine Advanced –Active Ingredients:
Dextran 70 0.1%
Polyethylene glycol 400 1%
Povidone 1%
Tetrahydrozoline HCL 0.05%
Inactive Ingredients: The bottle says: See Carton. This is the brand my wife uses and the carton has long since been thrown out. The Internet to the rescue.
Benzalkonium Chloride Solution
Boric Acid
Edetate Disodium
Sodium Borate
Sodium Chloride
Water (Purified)
The water I can understand; they need something to dilute down the active ingredients, but why’s the rest of this stuff in there if it’s too lazy to do anything?
2) Systane Lubricant Eye Drops–Active Ingredients: This is one I use.
Polyethylene glycol 400 0.4%
Propylene glycol 0.3%
Those are the only active ingredients; pretty stingy now that I think about it especially when they use less that half as much Polyethylene glycol as Visine.
Ah, but they make up for it.
Inactive Ingredients:
Boric acid
Calcium chloride
Polyquaternium-1 as a preservative
Hydroxypropyl guar
Magnesium chloride
Potassium chloride
Purified water
Sodium chloride
Zinc chloride
May contain Hydrochloric Acid and/or Sodium Hydroxide to adjust pH
Are you beginning to feel like this might have a certain character similarity to a patent medicine and yet it’s highly recommended by optometrists. But, remember that doctors used to recommend smoking.
Well, all I can say is that given these inactive ingredients we better get some pretty impressive crystals!
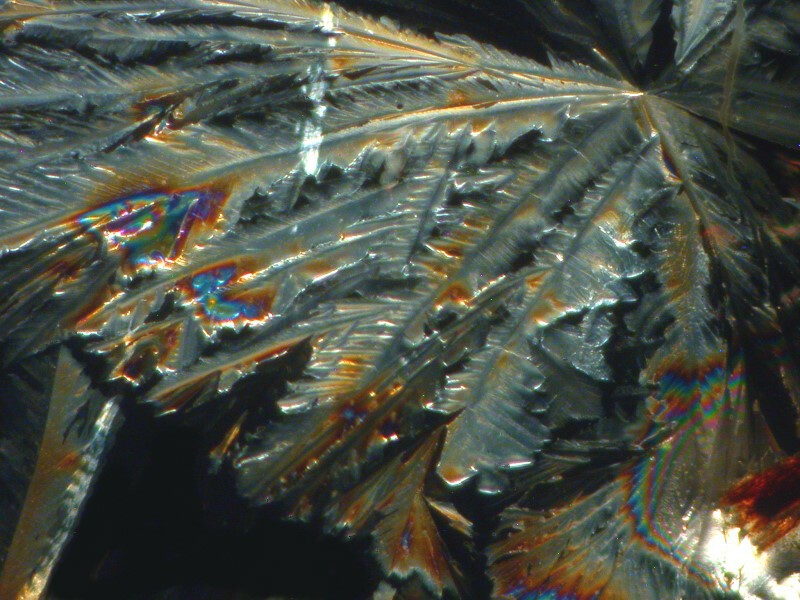
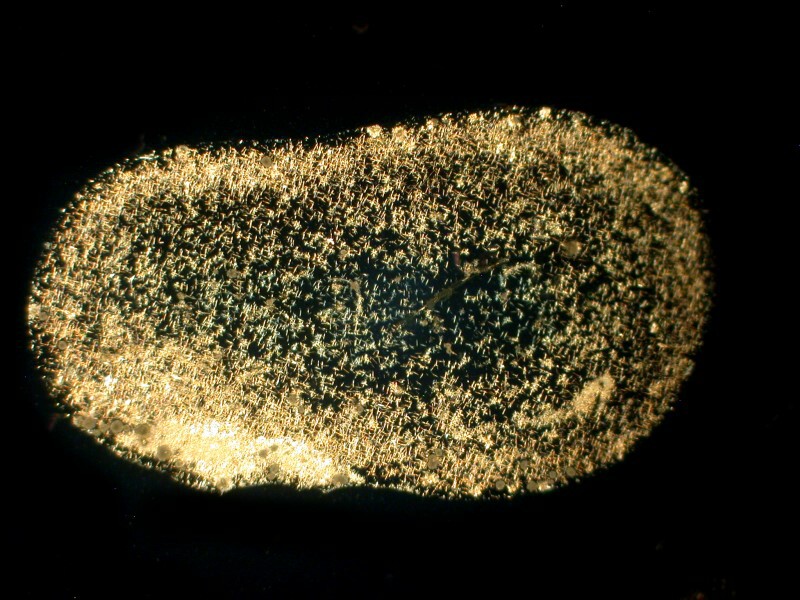
First Systane, 2 images showing some rather intricate lattices. (Polarized)
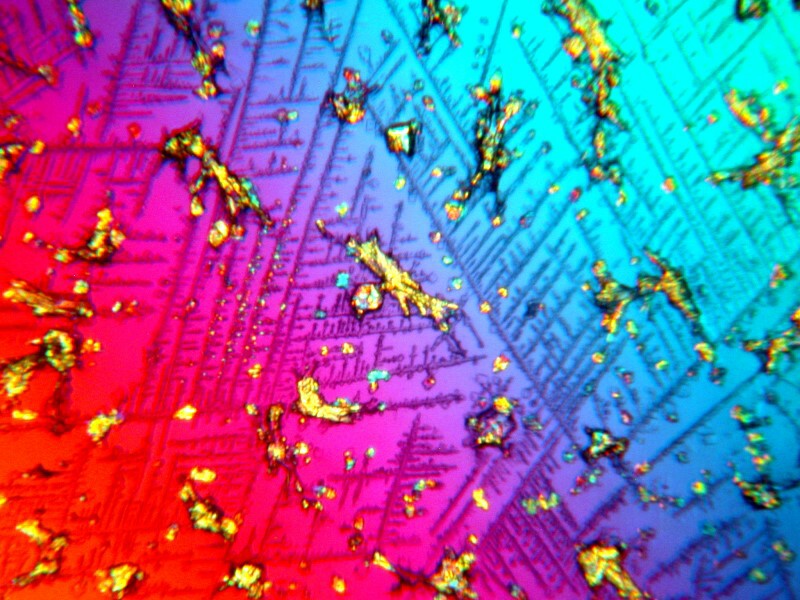
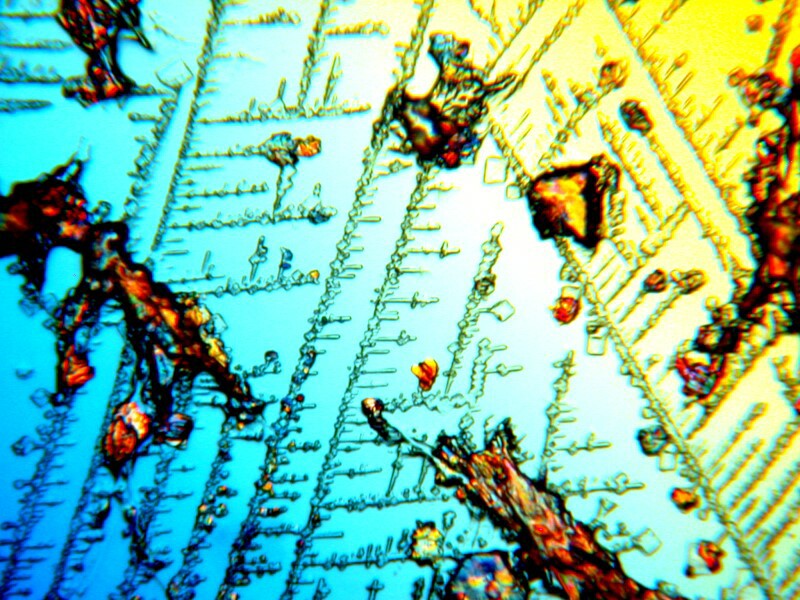
And a closeup of some of the larger crystals.
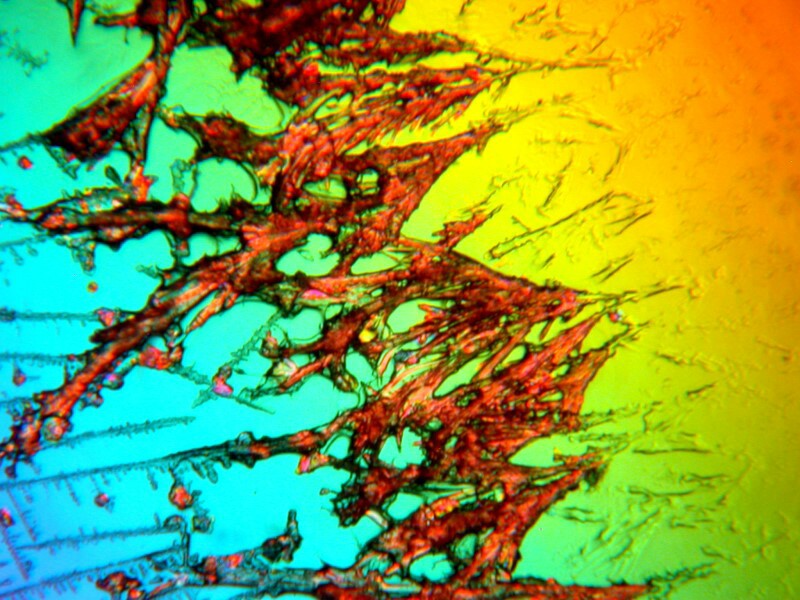
Not too bad; in fact, quite nice.
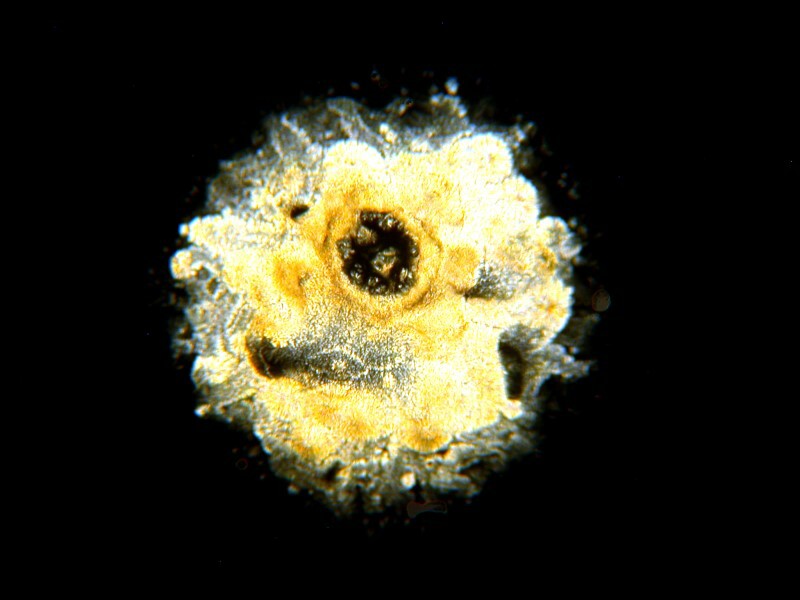
So, let’s see how Visine fares. As with most slides of crystals, there is a lot of variation and the interest depends significantly on which part of the slide you focus on. Of course, I want to show that my eye drops are better looking than those of my wife. So, here’s the first Visine image. (Both the images are polarized.)

Rather bland and ordinary.
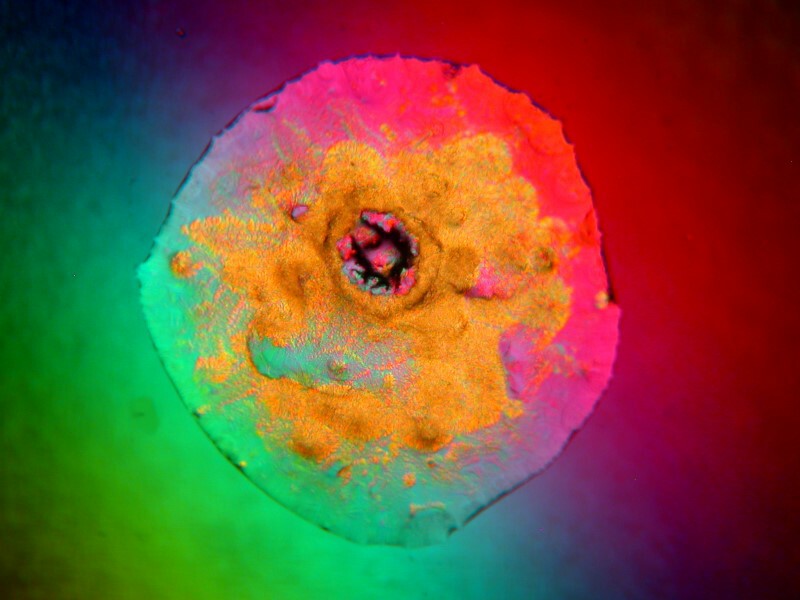
But I always try to be fair and I moved to another part of the of the slide and discovered this stunning feathery image which I must say beats my eye drops hands down.
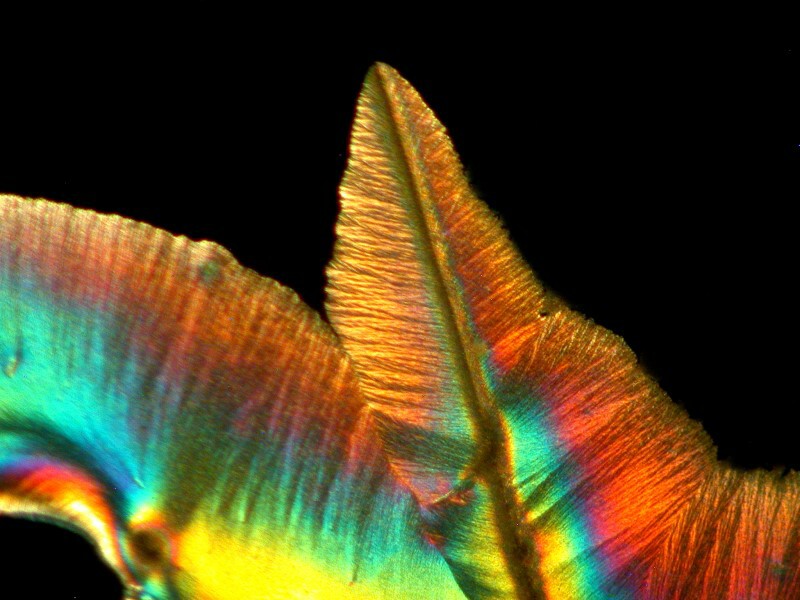
I told my wife after I processed this image that I now understand why her eyes are so much more lovely than mine.
All comments to the author Richard Howey are welcomed.
Editor's note: Visit Richard Howey's new website at http://rhowey.googlepages.com/home where he plans to share aspects of his wide interests.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
© Microscopy UK or their contributors.
Published in the December 2015 edition of Micscape Magazine.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk .