|
|
PHYSALOPTERA SQUAMATAE
A PARASITE OF ANOLIS
SAGREI THE COMMON GARDEN LIZARD OF CANCUN WALTER DIONI CANCUN,
MEXICO |
|
Note:
the pictures submitted in the following article had been taken between 2005 and
2008, with 3 cameras. Most of them with the DC3 MOTIC integrated into my
National Optical microscope (0.08 Mpx, resized by the internal capture program
to 640 x 480 px), others with a 2 Mpx webcam Logitech Quickcam Pro 9000 (supported
over the 10 X eyepiece with a homemade adaptor, and using only 60 % of the 2
Mpx sensor), and a few with an 8 Mpx Canon SX100is, that I borrowed from a
relative, focused at infinity and supported by hand over the eyepiece.
According to the needs of the best imaging, lighting used was Brightfield,
Oblique illumination, Darkfield or Rheinberg illumination. Backgrounds and dirt
have been cleaned with PhotoPaint. Color, brightness, and contrast, were also
adjusted with ACDSee, with which I also cut or resize. Net Image was used with
preference to control the noise. Some pictures are from
fresh material, but others have been taken from a pair of permanent
preparations stained with eosin and mounted in PVA-G. The preparations
were sealed with nail polish when they were made (2002), and remain perfectly
(perhaps giving a little more contrasty images) after 7 years. As always, those who are interested in
micro-invertebrate zoology should review their available textbooks, and the
literature recorded in the text of this article. |
INTRODUCTION
Some years ago,
a dull and weak blow at the sill of my window, interrupted my reading of the
local newspaper. A small lizard of about 8 cm in length lay motionless next
to the defense against mosquitoes. It was a “Brown Anolis”,
the most common and abundant lizard of Cancun gardens.
There are few
birds that visit our gardens, butterflies are rare, and most of the arthropods are little seen.
Therefore it is nice to see the small anoles sunning on a branch, running over
the white walls or between the colorful foliage, or extending its red gorge as a
warning.
Of course its
speed and restlessness makes difficult to capture them. So I watched carefully
the animal to prevent its movements and to try to catch it. My precautions
were useless: the animal was dead. Intrigued I decided to convert me to
forensic studies and perform its autopsy.
The result of it,
and the information I collected to understand and explain its death, form the
body of this article.
Although it is
recommended that everyone find their own on-line literature (as we used to do
in the normal libraries) always saves others a little work if reference links
are shared. So many references are attached in the text.
THE LIZARD
Anolis sagrei (Dumeril & Bibron, 1837) is a small grayish brown
lizard that greatly abounds and moves with impunity in the gardens of Cancun and
their surroundings. Apparently it is native to the island of Cuba, from where it
invaded, probably by human mediation, the Cayman Islands, and then the states
of Quintana Roo and Yucatán in Mexico (Álvarez-Romero, J., et al. 2005)
reaching the neighbouring Belize.
It also moved northward, invading the peninsula of Florida in the
United States from where it is spreading to Texas. There it competes (for
food, but also as a predator of small individuals) with the local anole Anolis carolinensis
(Green anole) that is being displaced (Campbell, 2002). It has been
reported even in Hawaii where it probably came at a time when the careless
handling of export fruit allowed easy transfaunation. (Goldberg &
Burgey, 2000).
Their taxonomic position is as follows:
· Kingdom: Animalia
· Phylum: Chordata
· Class: Reptilia
· Order: Squamata
· Suborder: Lacertilia
· InfraOrder: Iguania
· Family: Polychrotidae
· Genus: Anolis, Daudin 1802
· Species: Anolis sagrei, Dumeril et Bibron 1837
·
Subspecies: sagrei
(
It is worth
reviewing the definition of each category in a WebQuest. An article that
summarizes quite well these hierarchies is in Wikipedia (http://en.wikipedia.org/wiki/Taxonomic_rank)
Anolis sagrei
shows a clear sexual dimorphism. As it is common in the animal kingdom the male
is larger and has a distinctive
colouring, and special
attraction media, while the female looks more modest and inconspicuous.

Fig.
1 - Male on a tree, SX100is (x 10 zoom) Playa del Carmen, Quintana Roo

Fig 2
- The male torso, with insert of the unbent gorge (crop 1:1 of an SX100is 8 Mpx picture), Playa del Carmen, Q.R.

Fig.
3 – A whole Female (crop from an SX100is 8 Mpx picture), XeL-HA, Quintana Roo

Fig 4
– A closer picture of the same female
Since there are
many active herpetologists, and this species is relatively easy to raise in
captivity, searching by name with your browser you can find on the network many
pictures and even data for maintenance in a terrarium.
FORENSIC RESEARCH – THE
NECROPSY
Once dissected,
I found that the lizard’s stomach contained an entangled group of nematodes
(over 20 of all ages and sexes, which is admirable, given the small size of the
animal and the corresponding small stomach) many of them still clinging to the
stomach mucosa. Probably the poor animal could not feed and perhaps died a victim
of starvation and bleeding.
It is that, along
with the lizard have travelled their parasites. One of them, the nematode Physaloptera squamatae Harwood 1932, is
the subject of this note and its taxonomic position is summarized as follows:
Kingdom: Animalia
Phyllum:
Nemathelmintha
Class: Nematoda
Order: Spirurida
Family:
Physalopteridae
Genus: Physaloptera,
Rudolphi 1819
Subgenus: Physaloptera, Rudolphi 1819
Species:
Physaloptera (Physaloptera) squamatae Hardwood 1932
The fact that the structure of nematodes is very sketchy, leads almost all of the
authors to focus on presenting (even in drawings that define new species) only
the differences between the species described and others of the genus, so,
their concern is not to describe each entity in itself, but the differential details
(number of uterus; vagina position; size, shape, and type of development of the
egg, spicules and papillae in the male tail; structure and cephalic
organs, lips, teeth and spines of the mouth; and relative size (and relative
position) of the different organs. (See for example the description of the then new species
Ph. Buteonis.
An abstact can be found here http://www.jstor.org/pss/3223499)
Here of course
we are interested in the alternative strategy: to show visually all possible
details of this species’ anatomy.
GENERAL BIOLOGY
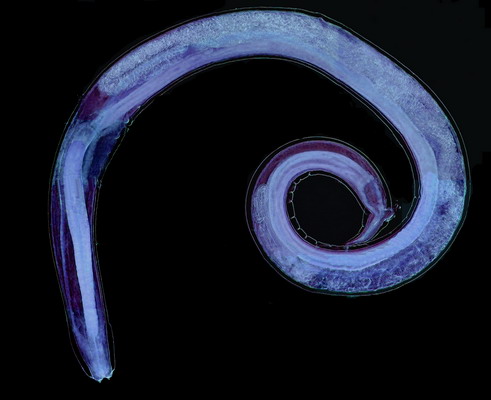
Physaloptera squamatae is good sized, the adult female I illustrate here (fig. 5) has an approximate total length of 10
mm and a
maximum width of 0.36 mm. Corresponding measurements for the male (fig. 42) are length = 7
mm and width0.3 mm. The younger female (fig. 25) measures
6 mm in length, and is 0.2 mm in width.
Apart from the
body wall, which is a circular muscle coat, wrapped in a flexible cuticle,
internal organization consists of a central digestive tract with a relatively
simple pharynx followed by the intestine, finished, near the tail, at the anus
in the female, and at a “cloaca” (the
organ that brings together the digestive and reproductive system) in the male.
The nervous system consists of a peri-pharyngeal ring (which acts as a brain
coordinator) and sends a dense package of nerves to the “head”, and two posterior
longitudinal nerve chains, included dorsally and ventrally in the muscle wall.
( see fig. 6
)
There are no
special systems dedicated to circulation or breathing. The small diameter
of the worms and the gas permeable cuticle acts as a respiratory
organ. Since the pseudo
cœlom has no
divisions, the simple wave motion that characterizes these animals generates
the necessary flow of fluids.
And the
spirurida excretory system is very simple consisting of longitudinal excretory
tubes that gather together in a inverted U shape at the level of excretory
pore.
A much more
comprehensive description, well illustrated, for anyone really interested in
the topic can be found in http://parasitology.informatik.uni-wuerzburg.de/login/n/h/0929.html#Fig-11
The reader unwilling to follow such a detailed description should check
the pages dedicated to nematodes of the excellent guide to practical zoology,
published online by Richard Fox of Lander University
http://webs.lander.edu/rsfox/invertebrates/ascaris.html
http://webs.lander.edu/rsfox/invertebrates/cephalobus.html
Structures
detectable with the imaging media at my disposal will be presented below for
both the male and female of Physaloptera
squamatae.
Preparing nematodes for observation - Nematodes are generally fixed by immersion in 70%
alcohol heated to boiling. So I took a test tube a quarter full with
alcohol, and (of course pointing the mouth of the tube in the opposite
direction from my body) I warmed it with great caution.
Alcohol has the
bad habit of boiling explosively, projecting fluid at a distance. It is
best to move repeatedly and slowly the tube through the flame several times
until it reaches the right temperature.
When just
beginning to boil, I picked up the nematodes with a brush and soaked
them in the liquid.
Normally the nematodes are
immediately stretched, setting straight, or slightly curved. The Physaloptera refused to respect this
custom and the longest died while maintaining a contorted shape.
To observe
nematodes it is best to clarify and mount them in glycerin. This is a method
that, with little effort, but careful work, provides excellent results. Indeed
it is the professional technique.
From alcohol 70,
nematodes are passed to a solution of glycerin, 5% - 10%, in 96% alcohol. It
is best to place them in a wide, shallow dish and place this inside a container
(there are many small plastic containers with a width of approximately 10 cm,
at least in one direction that could be useful) loosely covered, so as to allow slow
evaporation of alcohol. Use enough solution so that the alcohol evaporates
leaving the specimens still covered with
enough glycerin. Then move them to the final container with pure glycerin. Do
not forget to include a label with the corresponding data, written in indelible
" China” or “India” ink, or with soft graphite pencil.
To see them, put
a drop of glycerin on a slide, including a specimen. Add two or three strands of
plastic, or bits of plastic film or paper, or thin aluminum foil, or small
pieces of a broken coverslip, or any material of adequate thickness to allow a good compression of the
animal without crushing it too. Cover with a coverslip, with care not to
include air bubbles, and, placing an absorbent paper on the preparation, pass
the back of your hand on it in order to expel excess glycerin.
The preparation
is now
ready for the 4x to 40x
objectives.
But many details may require immersion oil. Therefore it is best to seal the preparation.
Place a drop of
nail polish on each corner of the coverslip and let dry for half an
hour. With a soft cloth, damp (not wet) in 96% alcohol clean the remaining
glycerin around the coverslip. Repeat operation after a few seconds to ensure a
good cleaning of the
slide. Then
seal the four edges of the coverslip with nail polish. After a few hours,
apply a second layer. This will provide a definitive preparation. Label it
immediately.
It is always
better to keep these preparations flat. Do not use common slotted boxes. In preparations kept vertically
the specimens can move slowly and
finish against one edge of
the preparation.
The images I'll
present here were taken from the better fixed specimens. Most correspond to 3 individuals: a juvenile female, an adult female and an adult
male.
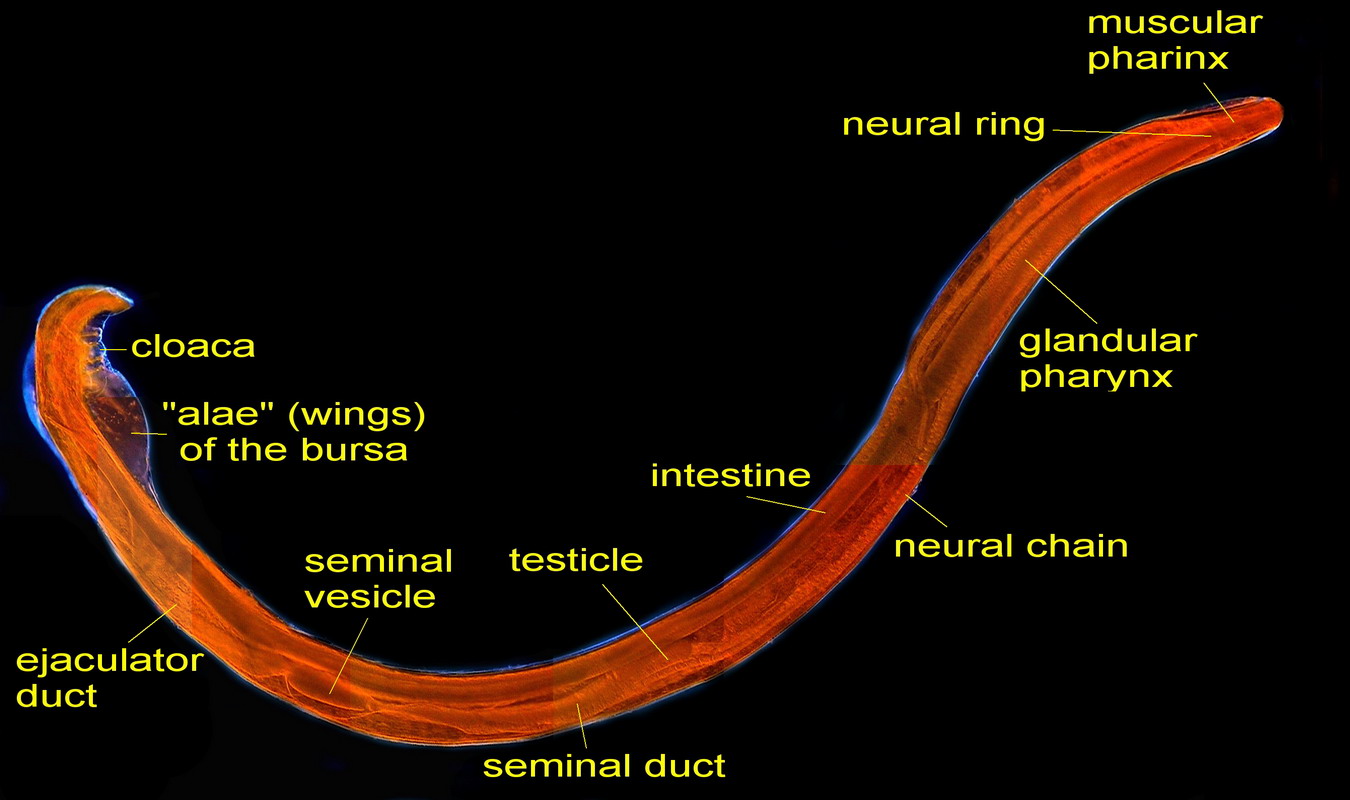
THE FEMALE ANATOMY.
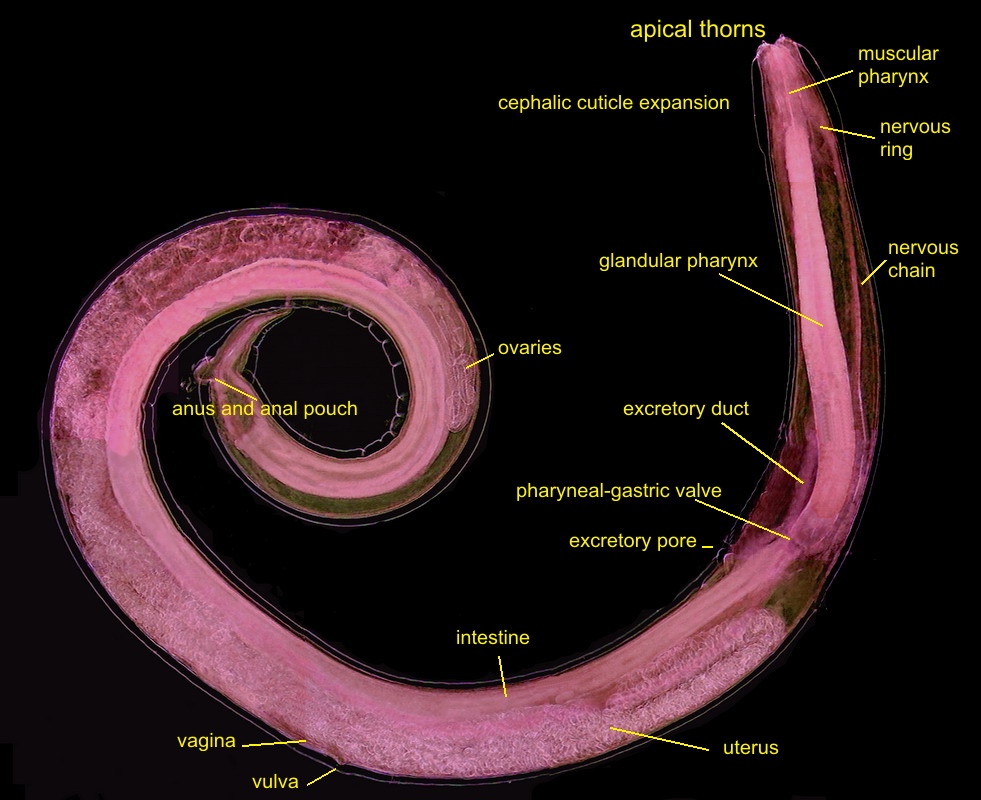
Fig 5
- A labeled picture of a PHYSALOPTERA female. Click on it to see a non labeled one.
The previous image is a mosaic of 10 pictures
taken with the 10x objective and the DC3 camera and finally composed in PhotoPaint. Labels indicate the most
important organs. Both pictures were reduced from the original mosaic of 3 Mpx.
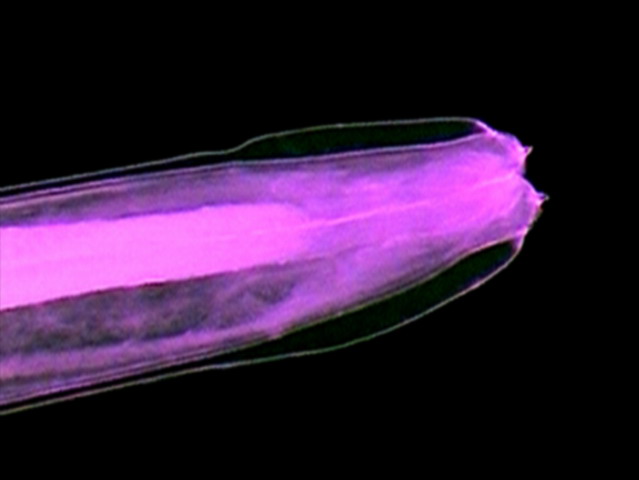
Fig 6
- Crop of a picture with the 10x objective
seen here as a difuse cone surrounding
the pharynx
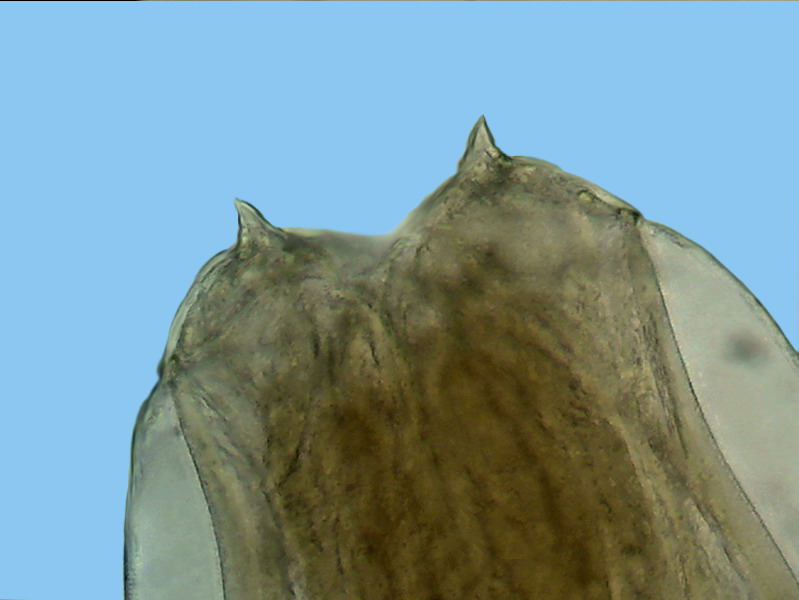
Fig. 7-
dorsal
view of the head, showing the two major terminals thorns. x40, Logitech

Fig. - 8 - lateral view of the “head” showing
the cephalic cuticular expansion and the peri-cephalic collar that helps the
animal, as if it were a suction cup, to adhere to the intestinal wall. Also look at the
nervous peri-pharyngeal ring, connected to the deirids - Logitech
|
|
|
Figs. 9 -10 - Deirids, left and right, DC3,
x 100
Deirids are
characteristic sensory organs of the nematodes in the morphologically defined Secernentea. The nematodes were once
divided taxonomically, based on criteria of morphological similarity in two
groups (Secernentea and Adenophorea), the adenophorea lack deirids.
However the current use of the biochemical
markers, as fractions of the RNA, has destroyed the apparent homogeneity of
these divisions, and currently the classification is very different. Anyone
wishing to initiate a review of this subject can consult Wikipedia articles on
both taxonomic categories. This work is an example of the problems that face
modern taxonomists:
A.Euyalem and M Blaxter - 2003 - Comparison of biological, molecular and
morphological methods of identification in a set of Panagrolaimus cultured species isolates. -
Journal of Nematology 35
(1): 119-128.
Five populations, all morphologically
assigned to a single species (ie. that they could not be
differentiated based on morphological characters) had to be separated into two different species since the study of its RNA showed that they were
genetically different. There is an abstract thereof published in http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2620612/

Fig. 11 - Another picture of the thorns
combining two shots with CombineZP
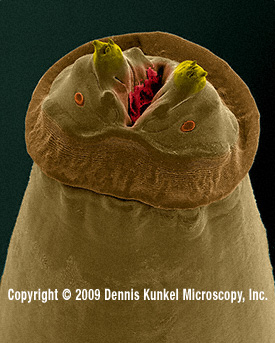
Fig.
12.
Anterior end (“head”) of a parasitic Physaloptera
from an American "racoon", photographed
with a scanning electron microscope, approx 70?x, by Dennis Kunkel, whom we thank for permission
to reproduce it. (For those which do not
yet know Mr. Kunkel, his site (see the address in MICSCAPE Links front page) is
full of images of the same quality, of many different biological entities. You
must visit it.) Both ovoids below the spines are the anphids, sensitive
structures considered of high taxonomic value.
It seems clear that the spines and the cephalic
collar, plus the muscular pharynx sucking action, must injure the gastric
mucosa. A study of the injuries inflicted by another species of Physaloptera, you can see in:
http://www.paru.cas.cz/folia/pdfs/showpdf.php?pdf=20977

Fig. 13-The
cuticle that forms the cephalic expansion is crisscrossed by many thin
transverse striations, as noted in this optical section, DC3

Fig. 14
- The picture shows the surface of the cuticle with the 100x OI objective,
showing the surface grooves. Cropping 1:1 image from 1.3 Mpx. picture.
Logitech
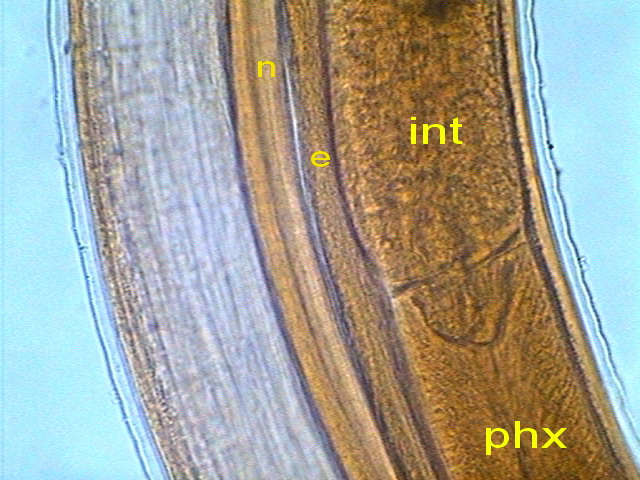
The outer layer of the intestine consists of a
epithelium with very uniform cells.
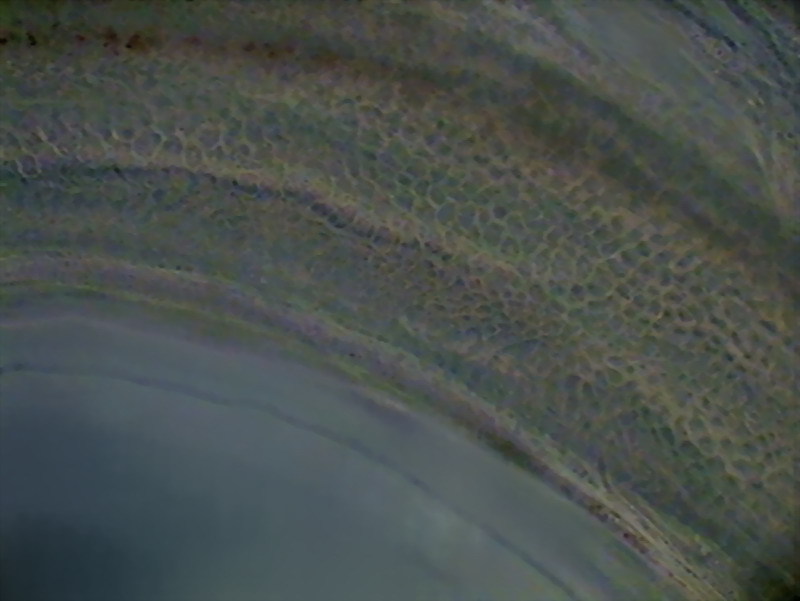
Fig16 - surface of the intestine - Logitech
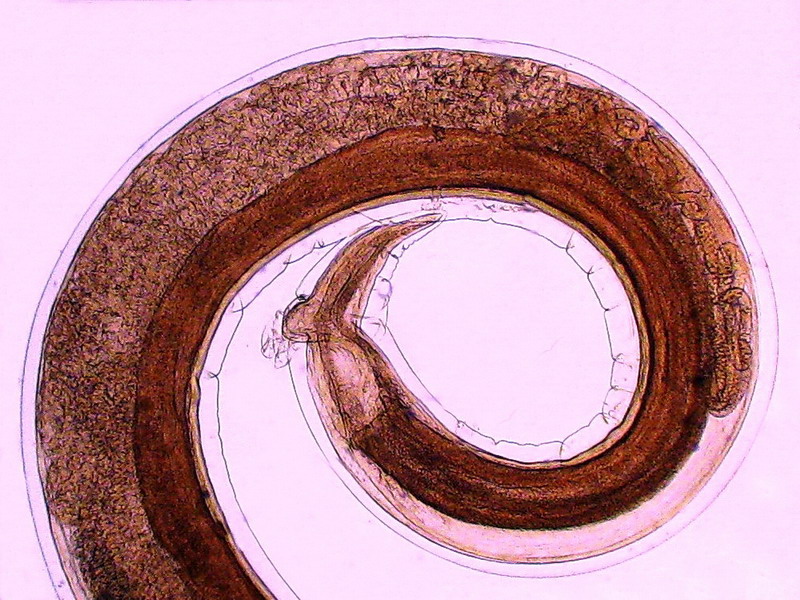
Fig.17
- the tail of an adult female (Canon SX100is, 8 Mpx) objective x 4 (clipping 1:
1)
The intestine opens abroad at a short distance from
the caudal end, through an extensive and folded anal pouch. The pouch is shown
in more detail in the following pictures.
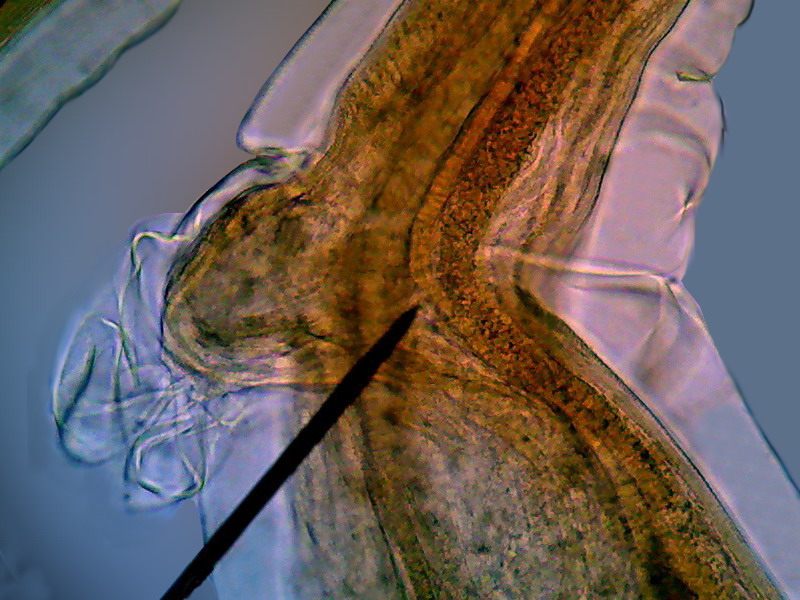
Fig. 18
- A detail of the previous picture. 40X, Logitech
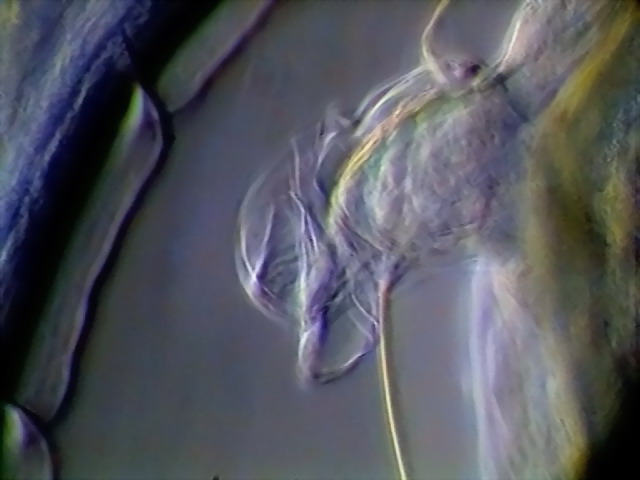
Fig. 19
- The same with oblique Rheinberg lighting, DC3
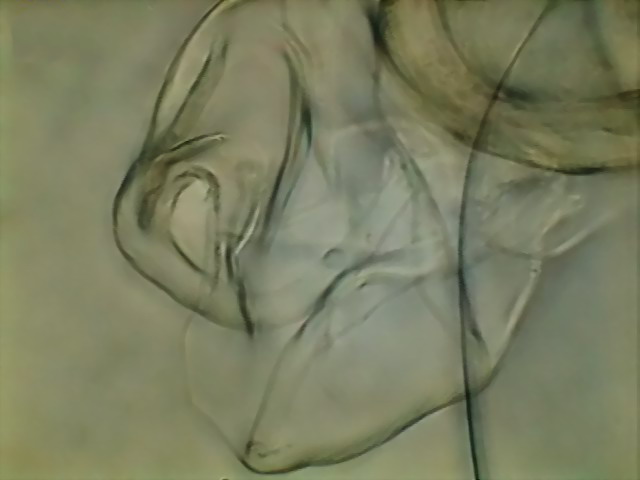
Fig. 20
- 100x, 5 images, combined with CombineZ5 - DC3
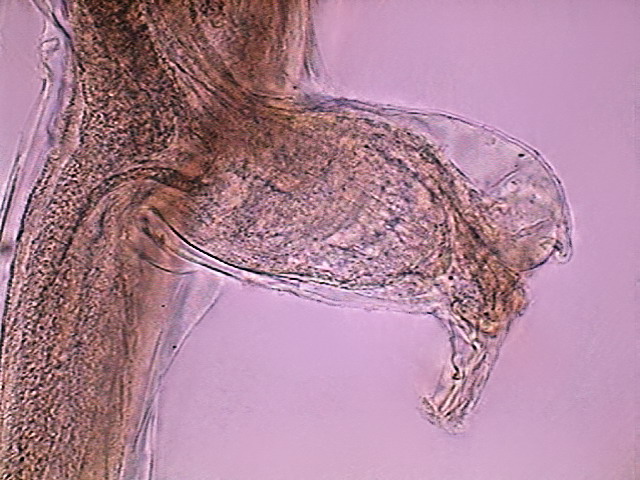
Fig. 21
- The anal pouch of another female, DC3, x 10
Fig.
22 – frontal view of the tail of the younger female
It shows only a very small pouch (p) and some rays can
be seen that could be perhaps interpretable as muscles to handle it. As the
picture is not conclusive I put it here only to arouse the interest of some
future researcher.
In my mounted
specimens the excretory system is difficult to
identify. (but see Fig. 15) I could acquire only
one image of the excretory pore.
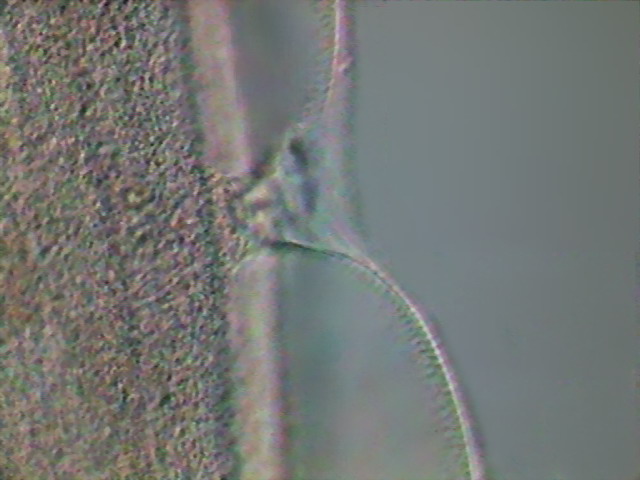
Fig 23- excretory pore - DC3, x40.
Two neural
chains run along the body, one in dorsal position, the other ventral.

Fig. 24 - oblique illumination, x40 – one
of the neural chains - DC3
The female reproductive system in Physaloptera is quite complicated. Much of this description is based on a young female, because
it was not so full of eggs, and allowed a better study of its structure.
I superimposed over a picture of it, taken with the x4 objective, (Fig 25) several boxes that correspond more or less (they are bigger) to
the levels where the descriptive pictures were taken, to make more clear the
basic layout.
Sexual organs are arranged in a
sequence as follows: ovaries, oviduct, (seminal receptacle), uterus, vagina and
vulva, that opens dorsally abroad usually a little ahead of half of the body. There are
multiple ovaries. The first portion of them, where the oogonia (primordial sexual cells) are visible is the germinal zone, it is continued by a growth zone, were the oogonia develops
into oocytes (ovocytes). And they go
through the oviduct to the first part
of the uterus, which function as a seminal receptacle. As ovocytes pass through,
they are fertilized and enter the uterus as eggs.
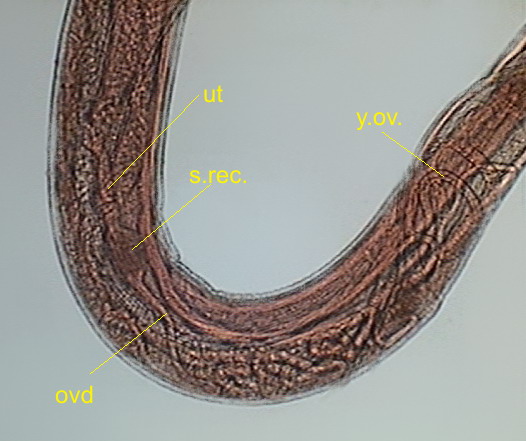
Fig 26 – .
y.ov - young ovaries, ovd - oviduct, s.rec. -seminal receptacle, ut – uterus
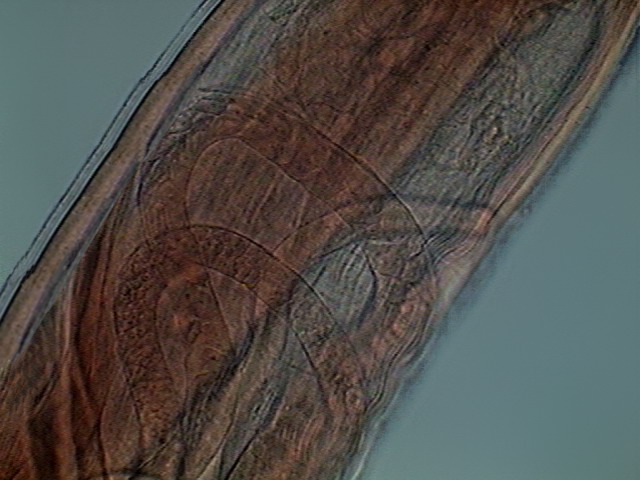
Fig. 27 – Ovaries, germinal zone of young
female, with visible oogonia
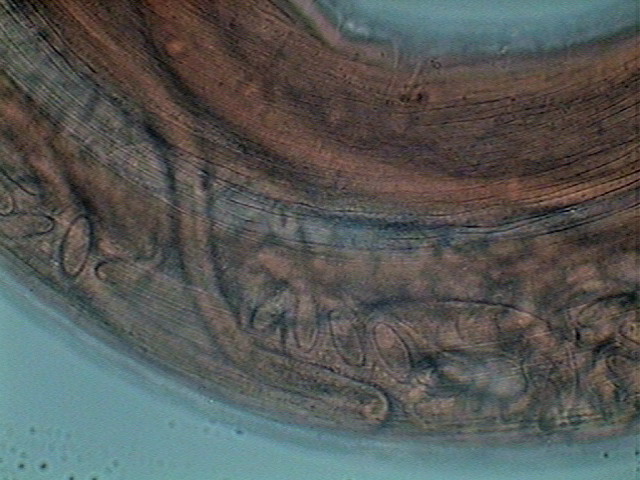
28 – Void oviduct
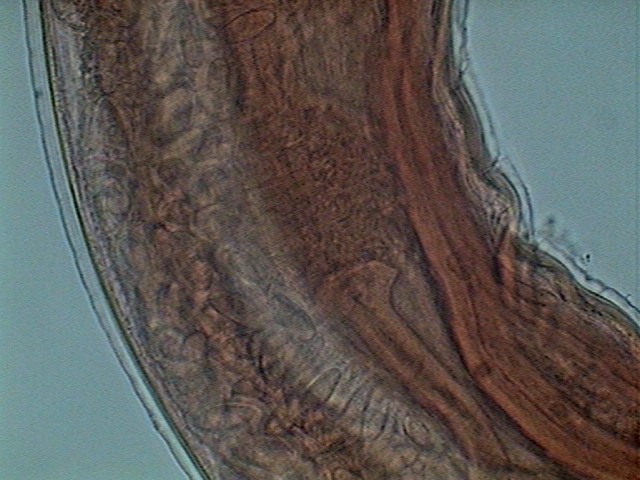
Fig. 29- Oviduct, Seminal receptacle and Uterus. Young female

Fig. 30 – Germinal zone, adult females.
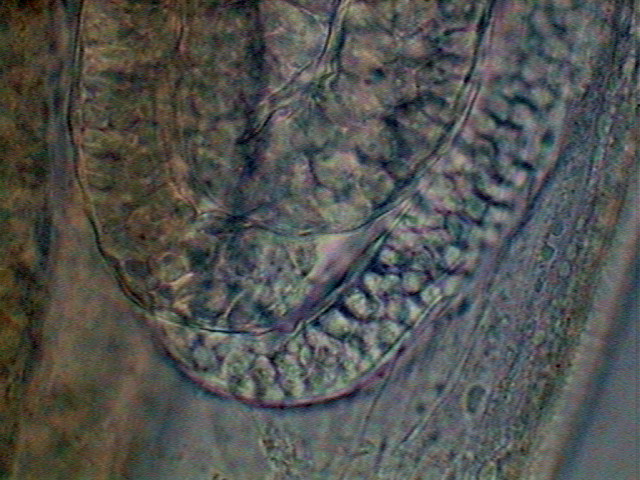
Fig. 31 – End of germinal zone start of the
growth zone, x100
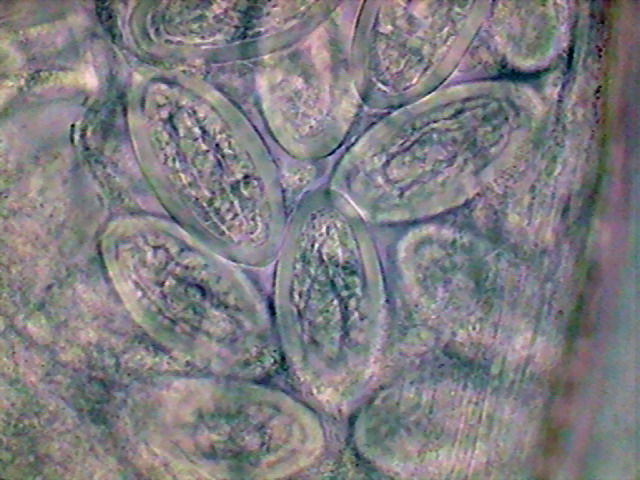
Fig 32 – Segmented eggs x100
Eggs with
embryos are released in the host intestine and go out with the droppings. The mature
eggs have inside a developed larva (larva 1 = L1). They measure aproximately 50
µ in length,
are 23 µ wide and have a shell of 3 µ. I tried, but was impossible, to measure the
length of the L1, it is not only contorted but folded in several planes as you
can see in the transverse optical sections of fig. 34.
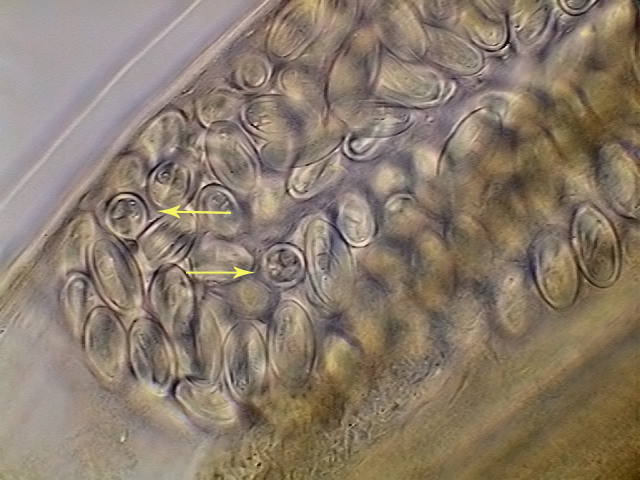
Fig. 33. - Eggs with already developed
larvae. A couple of eggs are seen in optical cross section. X40, DC3
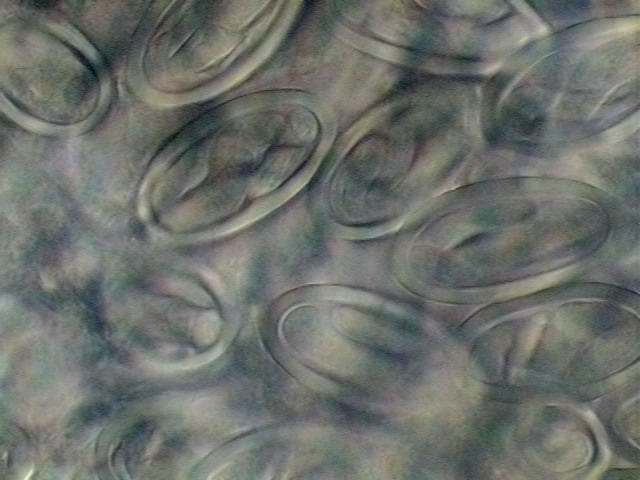
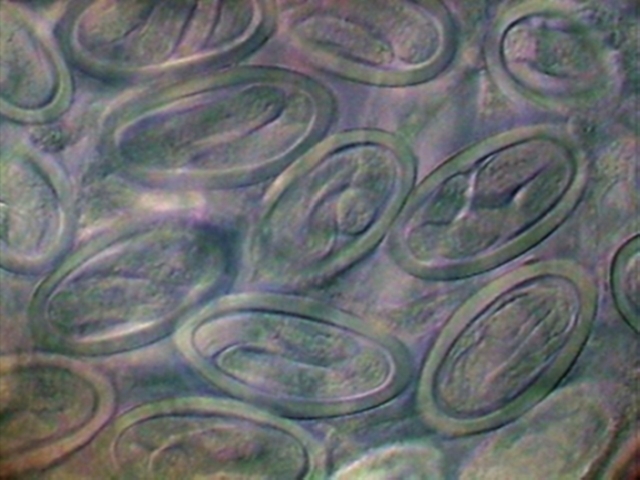
34. Two areas of the
uterus, with fully developed L1 larvae. DC3, 100
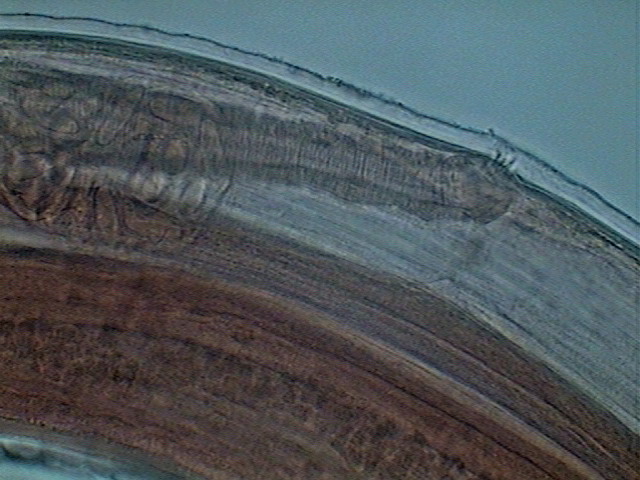
Fig. 35 - Vagina (heavely muscularized) and
vulva - young female. x40, Logitech
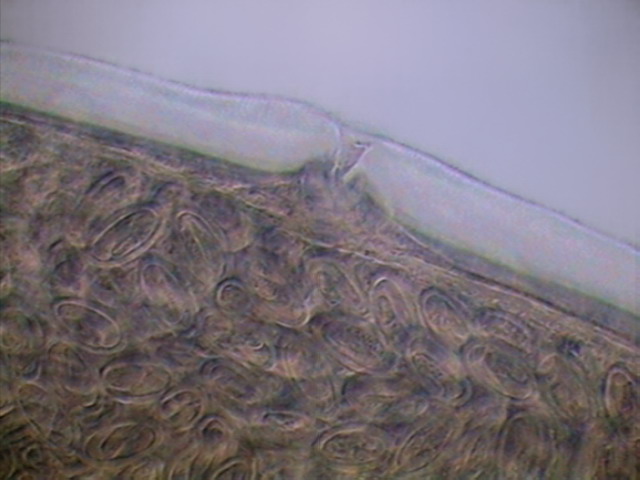
Fig. 36 - Vulva and uterus, with mature
eggs. Adult female. x40, DC3
Later
development requires the consumption of a stool by an insect, in whose hemocele
the larvae appears a few weeks after infection. L1 molts twice, and becomes L3,
which clings to the intestine of the intermediary host. Lizards eat these
insects, and free the L3 in their stomach where larvae suffer another two molts
and develop to adults, beginning a new cycle. I suspect that the larvae in my
garden must be hosted by ants, or by the juveniles of the American cockroach which
are the unique insects with enough abundance, and permanent presence, as to
guarantee the closure of the cycle.
Unlike the male which
has a tail with a defined hook-shape, the tail of the female is relatively
straight and gently tapered.
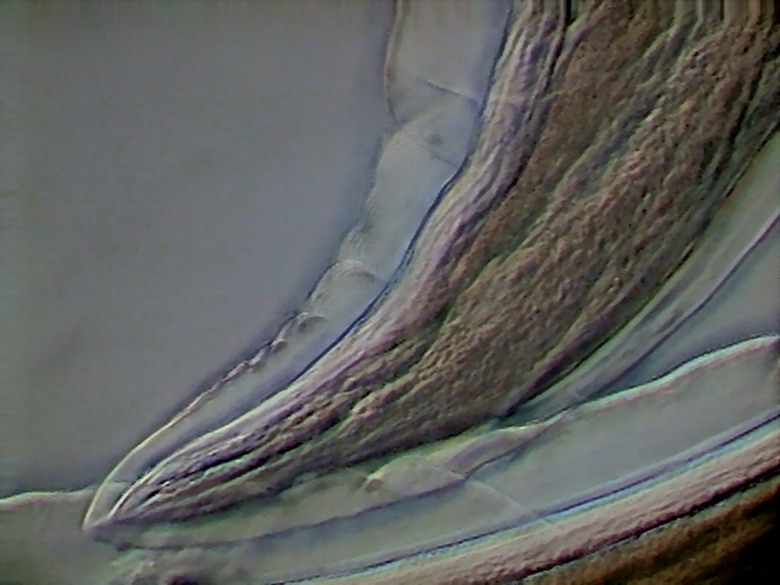
Fig. 37 – caudal end of adult female,Rheinberg oblique, x40,
Logitech
In the free nematodes,
especially the aquatic ones, freshwater or marine, there are caudal glands which
download an adhesive secretion through a terminal pore. This has an obvious
ecological value as this allows the nematode to cling in place even in running
waters. According to my Hyman textbook, and Internet references, parasitic
nematodes shouldn't have caudal glands, and therefore wouldn't have a terminal
pore. It is however difficult to interpret certain images (such as that added
below) without thinking that they show evidence to the contrary.
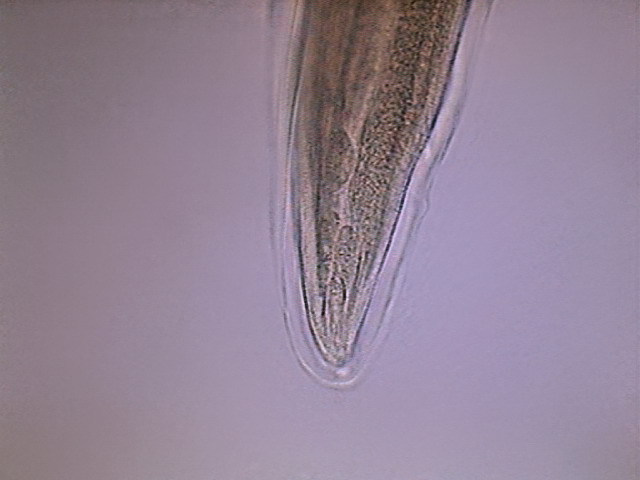
Fig. - 38 - Tip of the tail of another
specimen. It could be little doubt that this shows secretory cells. The
excretory pore is suggested by the final clear point.

Fig. 39 - In this picture the pore, and
even the excretory duct, seems to show more clearly
THE MALE
The male is slightly smaller, and shows the
most interesting
characteristics of the family.
|
|
|
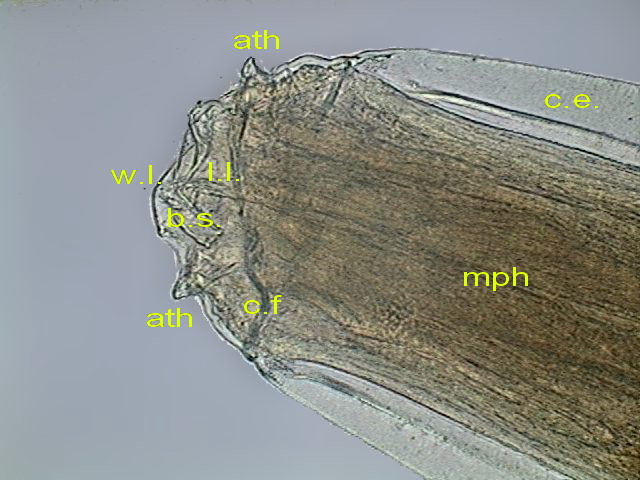
Figs. 40 - Right lip
(wl), Left lip) ll), slit to
the mouth (bs), spines (ath), muscular pharynx (mph), cuticular expansion (ce)
. DC3 (somewhat reduced from the 640 x 480 originals)
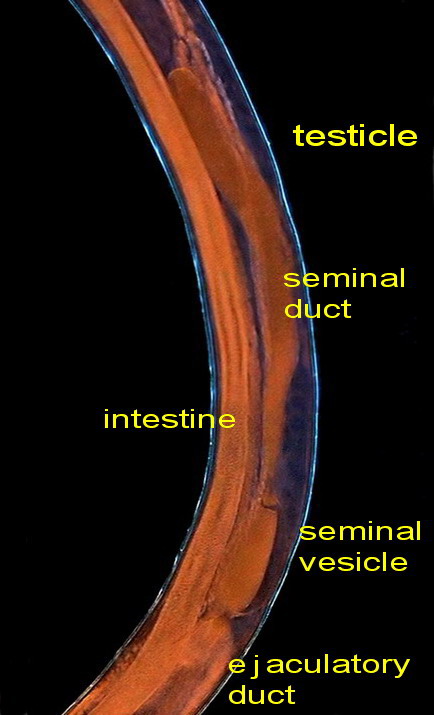
The sex organs are
arranged linearly: testicle, seminal vesicle, ejaculatory duct, cloaca,
spicules.
Figs. 41 – Male, full length. Click
on the picture to see a non-labeled version
|
|
|
Fig. - 42. The central section of the body
with the reproductive organs
These are pictures of the seminal vesicle and its
continuation with the ejaculatory duct.
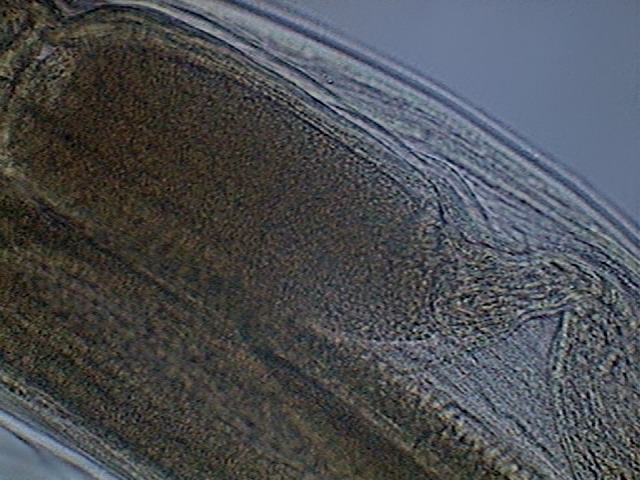
Fig .43 – seminal vesicle
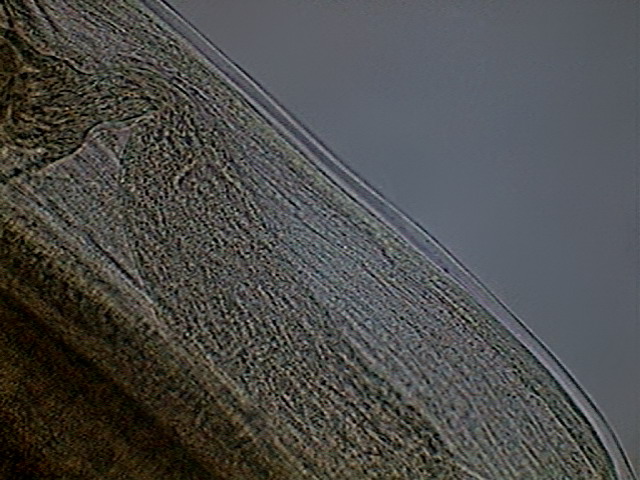
Fig. 44 - the ejaculatory duct
The ejaculatory duct
empties into the digestive system at the rectum, and this leads to the outside. The
cavity, composed by the end of both organs, is called a cloaca. The cloaca
is provided with a pair of spicules that apparently have a role in opening the gonopore
of the female. The cloaca is enclosed within an outer frame (or bursa) consisting
of two membranous wings (which Hyman calls “alae”), transparent and slightly
ornamented. Four "pedunculated papillae" on both sides of the
cloaca help provide rigidity and functionality to the copulatory bursa.
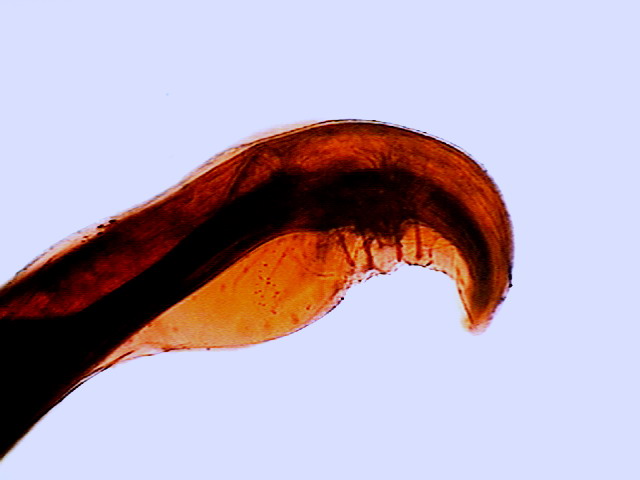
Fig. 45 - Tail from a specimen stained
with eosin. One can see the wings (alae) of the copulatory pouch, the cloaca
and the stalked papillae. The focus is made on the upper surface
(proximal) of the worm – DC3
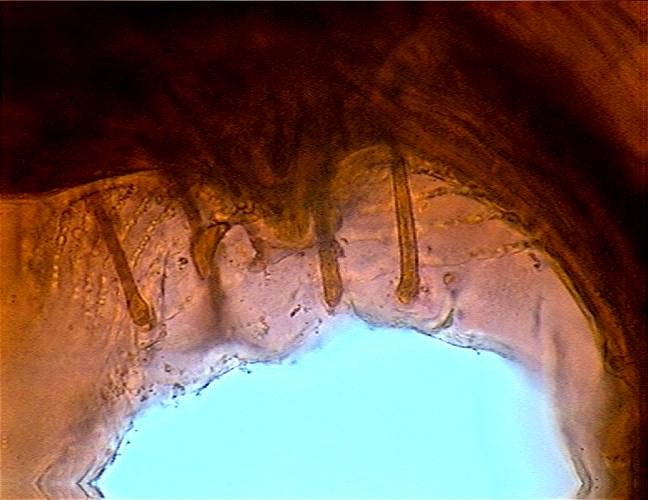
Fig. 46 - Cloaca and stalked papillae at a
high magnification, with a partial view of the ornamentation of the alae. DC3
|
|
|
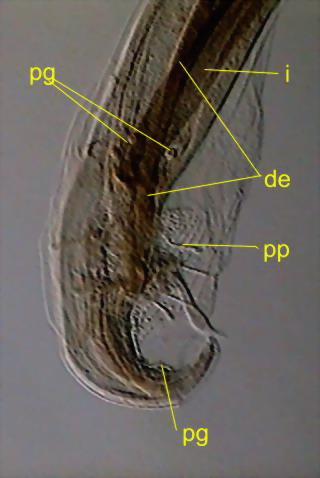
Fig. 47 - caudal end and copulatory
complex. de - ejaculatory duct (comes from the seminal vesicle, ends in the
cloaca), i - intestine, pg - sessile glands, pp - stalked glands. There are also
seen the alae and the spicules. DC3

Fig. 48 - Spicules and glands with greater
detail. spc, spicules, sp, one sessile papilla pp, pedunculated papillae, c, cloaca, a, alae DC3
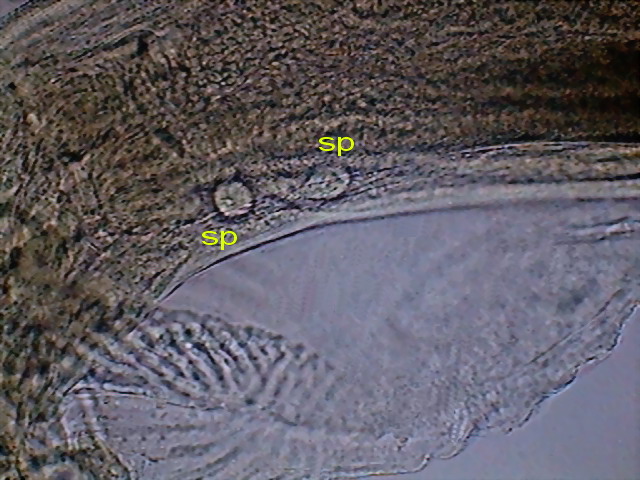
Fig. 49 - A couple of pre-spicular sessile genital
papillae (sp) DC3
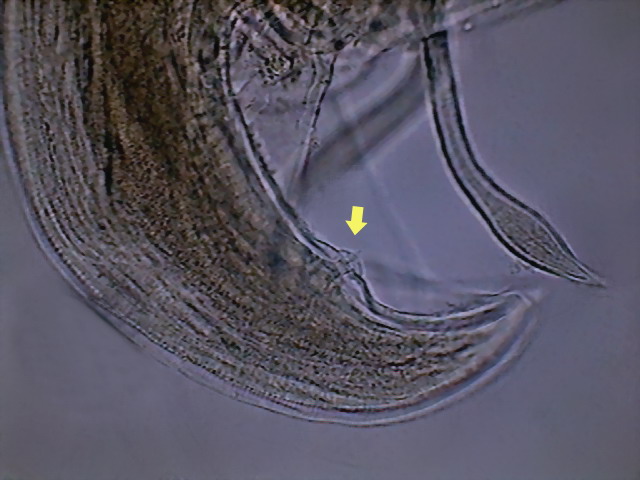
Fig. 50 - And, finally,
an image showing one caudal
sessile papillae (at least 3 other papillae can be spotted on my specimen, but I
have no references of how many or in which position are located those that are
normal for Physaloptera squamatae – Hyman
draws 6 post anal papillae for an unnamed species) DC3.
I think that
this article, that lay waiting for so many years on my hard disk, is almost
certainly my last one on parasitic nematoda. But, for the parasitologists-to-be
that could be amongst the readers, I recall that I published a short
series on the nematoda and other parasites of the cockroach Periplaneta americana.
http://www.microscopy-uk.org.uk/mag/artjun05/wdparasite.html
http://www.microscopy-uk.org.uk/mag/artjul05/wdparasite2.html
http://www.microscopy-uk.org.uk/mag/artaug05/wdparasite3.html
http://www.microscopy-uk.org.uk/mag/artsep05/wdparasite4.html
http://www.microscopy-uk.org.uk/mag/artoct05/wdparasite5.html
and one on the
parasites of the “tropical gar” a “living fossil” from Mexico
http://www.microscopy-uk.org.uk/mag/artjun09/wd-pelegarto.html