|
Some Further Reflections On Micro-Technique by Richard L. Howey, Wyoming, USA |
It is highly important that amateurs share tips, observations, and suggestions with each other, especially regarding living micro-organisms or concerning micro-structures of larger living or preserved creatures. Much of the published information on such topics is highly technical and is often in journals or books which are extremely expensive and sometimes difficult to gain access to. This is the reason why Micscape (and other microscopy and natural history websites) provide such an important forum and reference resource for amateurs. Maurice (Mol) Smith and David Walker deserve a standing ovation for their long-term effort and support in providing all of us with this splendid resource. So, get up from your computer and give them a long round of applause. We should never take their hard work for granted, so send in an article, some notes, some images with a few comments or a small, medium or very generous financial donation. (Hey, Bill Gates and Warren Buffet–are you listening?) There is, of course, one other option; you can send in long, rambling, semi-coherent essays like I do.
Let me begin this one with a few more remarks on the subject of vital stains.
Vital Stains
As the name suggests, these are stains that are intended to act on the organism to provide contrast or differentiate specific structures without killing it or, at least, not immediately. The degree of progressive toxicity varies widely. Clearly, such stains must be employed in highly diluted solutions and, ideally, be allowed to be absorbed very gradually, although that is not always possible. Nature is always presenting us with challenges and, in this case, one quickly learns that what one organism tolerates well is highly disruptive to other species. This, of course, means that there are no simple, general protocols, but rather a series of suggestions to narrow the range of possible experiments one needs to try. The number of variables to be considered can be overwhelming and can even include not only the manufacturer of a particular dye, but even the specific batch.
1) I started with a sample containing tiny flagellates (Chilomonas), bdelloid rotifers, Paramecium, and Spirostomum intermedium. I used a series of 16 ml. test tubes and to the first one I added enough 1% Neutral Red to produce a distinct pink color. After 6 days, the flagellates and the rotifers were largely unaffected, whereas the Paramecia were heavily stained, but vigorous. The Spirostomum, however were grossly deformed, most being simply reduced to spheres, although a few were spheres with a “proboscis”.
2) To this tube, I added just enough of a 1% solution of Nile Blue to give it a light bluish tinge. One Spirostomum survived; all else–rotifers Paramecium, Chilomonas were dead.
3) Bismarck Brown traditionally has a record of relatively low toxicity. In this tube, the Chilomonas, rotifers, and Paramecia were all heavily stained but vigorous. The Spirostomum were distorted.
4) To this fourth tube, I added Janus Green B. This is a dye which I have found to have a rather high level of toxicity. In this sample, everything was dead except for one rotifer and one Spirostomum which remarkably was undistorted. There was a clear indication that the stain slowed the contractility of the Spirostomum and this is something that is certainly worth investigating further.
5) This sample was treated with the wonderfully versatile polychrome stain, Methylene Blue. Polychromes are often rather unpredictable since their “character” depends on a variety of impurities. The Chilomonas, rotifers, and Paramecia were all heavily stained. However, with Paramecium there was about a 50% mortality rate.
6) Toluidine Blue. Here my notes mention only Paramecia and they were well-stained and vigorous. This leads one to suspect that the other organisms may have also fared well, but such extrapolation without direct evidence is risky.
7) With Methyl Green, both Spirostomum and Paramecium stained well and were vigorous and there was even one specimen of Paramecium which was dividing.
A Second Series of Samples Using The Same Stains.
This material was taken from a bucket which had been sitting on the side of the driveway. It contained some bits of leaves from shrubbery which had been trimmed and a few grass clippings. It contained small hypotrichs, small ciliates, and some very tiny flagellates.
1) Neutral Red
Good Staining of the small hypotrichs and ciliates.
2) Nile Blue
Virtually all the organisms were dead; the only exception was some of the very tiny flagellates.
3) Bismarck Brown
The small ciliates and flagellates stained well, but not the hypotrichs.
4) Janus Green B
All the organisms were dead except for one quite small hypotrich.
5) Methylene Blue
The small ciliates were well-stained and were flourishing and, interestingly they seemed a bit hyperactive. When a foreign substance, be it a stain or some very dilute reagent, is introduced into a sample, such hyperactivity on the part of some organisms is not uncommon. This is an intriguing phenomenon which should be investigated further and is, I suspect, in some instances, related to hormesis whereby a small amount of a toxic substance can actually prove beneficial to an organism.
6) Toluidine Blue
The small hypotrichs and flagellates were flourishing.
7) Methyl Green
All of the organisms were flourishing.
Although this was a very small experiment, it does suggest that with protozoa, Nile Blue and Janus Green B are not very promising as vital stains.
Iodine
A weak solution of iodine in alcohol (tincture of iodine) can be purchased at almost any store which has a pharmacy. This reagent is very useful for a variety of procedures and is easy to use and can produce some very interesting results. Many microscopists prefer Lugol’s solution which has Potassium iodide added to an aqueous solution of iodine. This is available from biological supply houses. I would strongly recommend that you not try to use iodine crystals to make up your own solutions. In the U.S., iodine crystals have recently become more highly restricted as they rather readily give off vapor which can be highly toxic, so buy either the tincture or Lugol’s already prepared. If you have the alcoholic tincture, but you wish to use it as an essentially aqueous solution, this is easy to accomplish. Simply spread a drop of the tincture on a slide and let the alcohol evaporate. When it’s dry, you simply add a drop of the culture solution of the organism which you wish to treat with iodine.
Iodine has an affinity for starches which you can easily demonstrate by taking a scraping from a freshly cut potato and adding a drop of iodine. A wide variety of plants contain starches of various sorts so, for botanical microscopic investigation, iodine is a very useful reagent.
Destaining
Sometimes in the process of staining, one gets overly enthusiastic and uses a solution of stain that is too strong or one leaves the specimen in the stain too long. Sometimes, with certain stains and certain procedures, overstaining is recommended, because the destaining procedure if carefully monitored, can allow for quite subtle differentiation and can reveal detail that might otherwise be obscured.
The most common destaining reagent is Acid Alcohol which can be made up with 50 ml. of 35% alcohol and 3 drops of concentrated Hydrochloric acid. (CAUTION: This is a very aggressive acid which quickly attacks tissues and the vapors are dangerous to the eyes, nose, lungs and skin, so it must be handled with great care.) Once the Acid Alcohol solution is prepared, it is relatively harmless but, I wouldn’t recommend it for making martinis.
Destaining should be carried out under the microscope under constant observation. When the dedifferentiation is at the right point, you can stop the process by pipetting off the excess Acid Alcohol and adding a bit of distilled water.
A Few General Purpose Stains
The variety of stains and staining techniques and modifications of both is simply staggering and here I’m talking about just light microscopy and excluding exotic reagents or procedures for high-tech microscopy. However, for light microscopy, there are a number of standard stains which are quite common, fairly easy to obtain, and provide consistently good results.
1) Acid Fuchsin when used at approximately 0.5% concentration is an excellent cytoplasmic stain.
2) Basic Fuchsin is a very good nuclear stain for fixed material.
3) Methyl Green Acetic which is used as a 1% solution of Methyl Green with enough acetic acid to make the acid content 1%. The great advantage of this stain is that it is used on living organisms and tissue and is a powerful nuclear stain. As you would expect, the acetic acid produces some distortion and swelling but, if ones primary concern at the moment is nuclei, then the results are very good. I have had good success in staining and examining the nuclei of highly contractile organisms such as Spirostomum, Stentor, Lacrymaria, and Vorticella in spite of the distortion. Below, you can see a portion of a Stentor where the beaded macronucleus is clearly evident. The contractile fibrils are also visible.

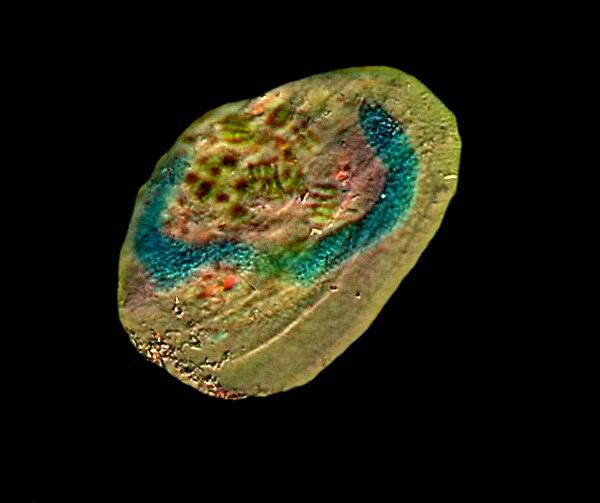
The next image is of the hypotrich Euplotes and here the long “C” shaped macronucleus can be clearly seen.
4) Methylene Blue is perhaps the most frequently used polychrome dye for general microscopy. Its impurities which determine its polychrome character will mean that you won’t always get consistent results even with the same batch of solution and the same organisms. Nonetheless, the results are often striking and reveal well-delineated detail.
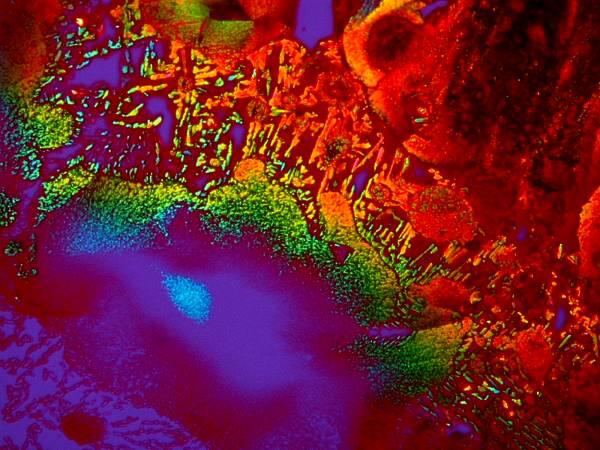
5) Orange G is an excellent cytoplasmic stain and is often used in conjunction with other dyes as a contrast stain. Just by itself, it is a delight to observe under polarized light. Be sure to rotate the analyzer as you are examining it and observe the dramatic color shifts. If you have the stain in an alcoholic solution (I use 70% Isopropyl alcohol) then you can spread a drop on a slide, place it under the microscope making sure that the polars are crossed and that you have a black field of view and, within a minute or so, you will be able to observe a microscopic fireworks display as the crystals form in front of your eyes. This is especially true when the Orange G is mixed with certain other substances. I’ll give you 3 examples here.

This is Orange G mixed with Rochelle Salts (Potassium Sodium Tartrate). These elongated crystals are quite typical of Orange G.
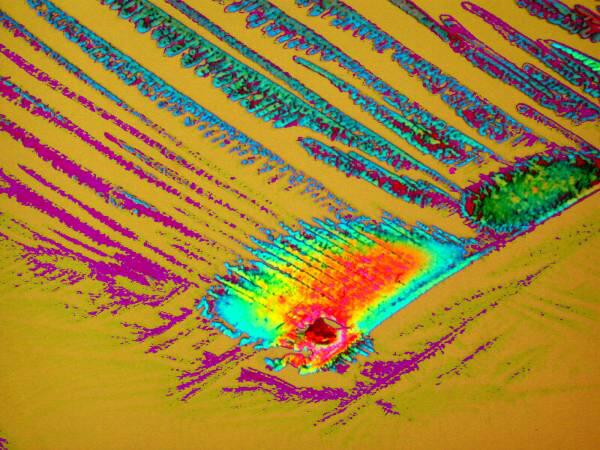
Both of these are a mixture of Orange G and a liquid toothache medication and although they are remarkably different both images were taken of the same slide.
6) Hematoxylin stains have long been a centerpiece of optical microscopy and over numerous decades, many different solutions and procedures have developed, many of them complex, demanding, and time-consuming often involving multiple steps an reagents. In many instances, good results can be obtained using a professionally prepared solution of Delafield’s hematoxylin the procedure for which is straightforward and provides quick results allowing one to assess the suitability of the stain and the technique for your immediate purpose.
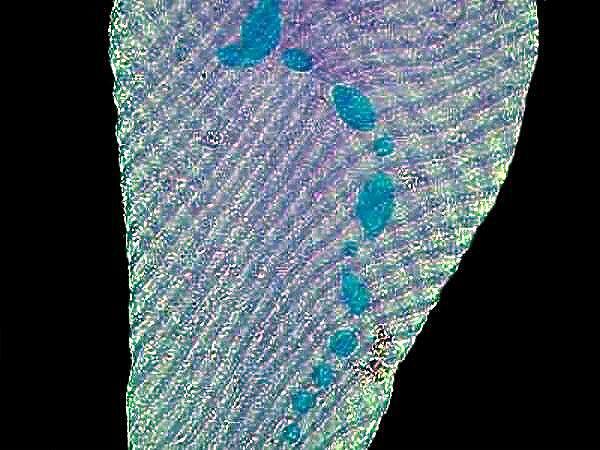
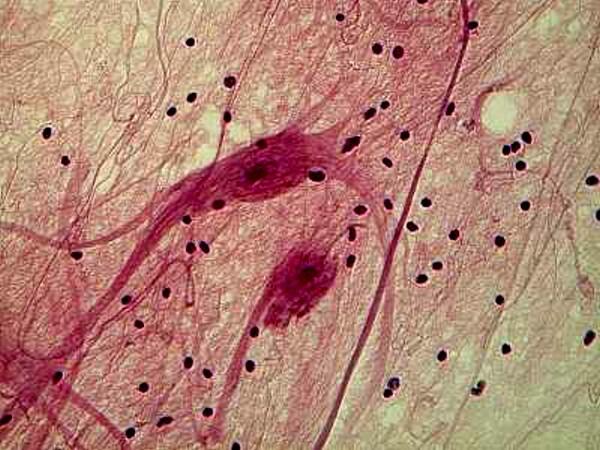
Hematoxylin stains are very often used to stain tissue. Here is an example of Ox nerves stained with Hematoxylin.
If you are serious about experimenting with micro-technique, then it is essential that you keep carefully detailed records if, for no other reason than to avoid discovering that you had already tried a certain technique which failed miserably and that it is once again a miserable failure. However, more importantly, such documentation can provide you with a record of successful experiments which you share with the readers of Micscape.
All comments to the author Richard Howey are welcomed.
Editor's note: Visit Richard Howey's new website at http://rhowey.googlepages.com/home where he plans to share aspects of his wide interests.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .