|
|
A Gallery of Benzoic Acid Photomicrographs (using
phase-contrast illumination) |
|
|
A Gallery of Benzoic Acid Photomicrographs (using
phase-contrast illumination) |
One of my favourite places is the
Museum of Modern Art (MOMA) in
New York. I have spent many pleasurable afternoons wandering
around the building, searching in vain for art that matches the beauty
of the architecture. For some strange reason, a red dot on a
plain white canvas, or a pile of plastic excrement on the floor,
doesn’t
inspire me to exclaim “brilliant, insightful – a joy forever”! I
have been told many times by art connoisseurs that the fault is
entirely mine. Bearing in mind that such a beautiful building has
been erected to house them, and the immense cost of such “works of
art”, there is obviously something that I am missing!
While taking the benzoic acid
photomicrographs of melt specimens for this article, it struck me that
the compositions and colours visible under the microscope, rivaled, or
even exceeded those in modern art creations. Of course, the
structures seen on the microscope slide are not produced by a human
artist, but by the laws of chemistry and physics. As the molten
state cools to the solid state, molecules with positive and negative
ends attract one another, and tend to arrange themselves in
three-dimensional lattices called crystals. If the cooling
process occurs slowly, there is time for large, perfect crystals to
form, whereas rapid cooling results in small, disorganized groups of
crystals.
I leave it to the reader to be the
“art critic” in this situation. Is it physics and chemistry, or
artists that produce the best “modern art”. You be the judge!
Benzoic acid is a white crystalline
solid with a melting temperature of about 122 degrees Celsius.
This low melting point makes it easy to produce a melt specimen by
placing a few crystals on a slide, covering with a cover-glass, and
heating gently over an alcohol lamp until the solid melts. Slides
prepared in this way cool to room temperature in about a minute.
It should be kept in mind that the MSDS safety document for the
compound states: “May be harmful if swallowed. May act as an eye or
respiratory irritant. May cause allergic respiratory or skin reaction.”
Benzoic acid C6H5COOH is
the simplest aromatic (based upon a benzene ring) carboxylic acid
(containing the COOH group). The structural formula and molecular
shape, (produced using HyperChem Pro),
can be seen below.
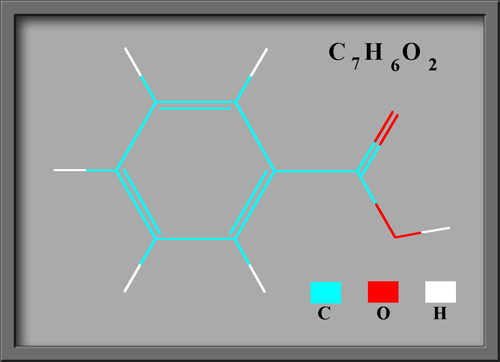
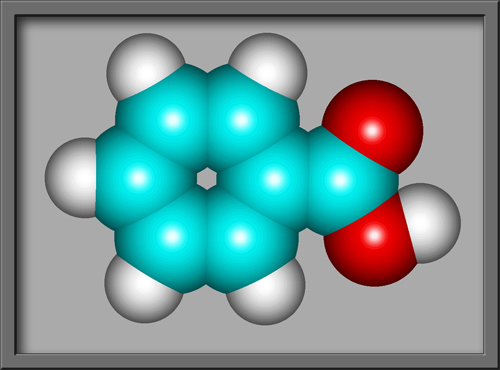
This compound is often used as an
anti-microbial agent in products like cosmetics, toothpastes,
mouthwashes and deodorants. Fruit products, beverages and
condiments may use benzoic acid as a preservative. In such
applications the quantity used, is of course very small, in order to
reduce the harmful effects mentioned above.
Benzoic acid melt specimens can be
examined under the microscope using polarized light. In this
article, however, phase-contrast illumination was used
exclusively. A Leitz 402a phase-contrast condenser and Leitz 25X
NPL Fluotar PHACO objective were used to form the images with a Leitz
SM-Pol microscope. (Since only one objective was used, it should
be noted that all of the images have exactly the same
magnification.) An article concerning the same compound
illuminated by polarized light will be published in a future issue of
Micscape.
Notice the unusual mottled pattern
in the image below.
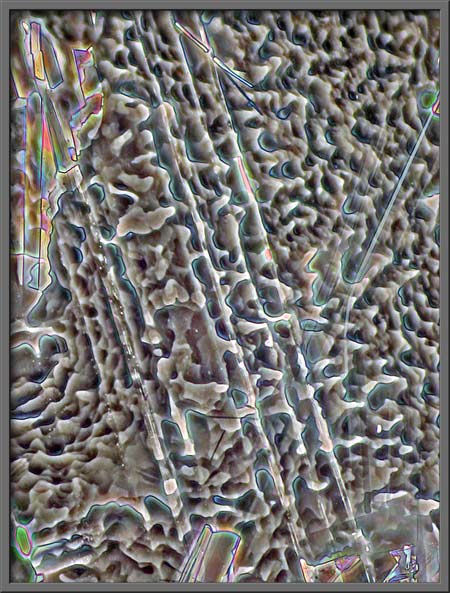
Post-processing of an image is
possible in Adobe Photoshop; I use the CS version. One technique
is to use the “Inversion” tool
to alter the colours displayed in the original image. The diagram
below shows a colour spectrum before and after inversion.
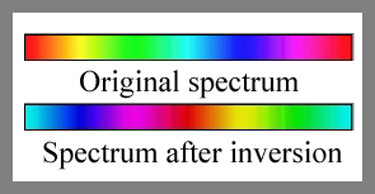
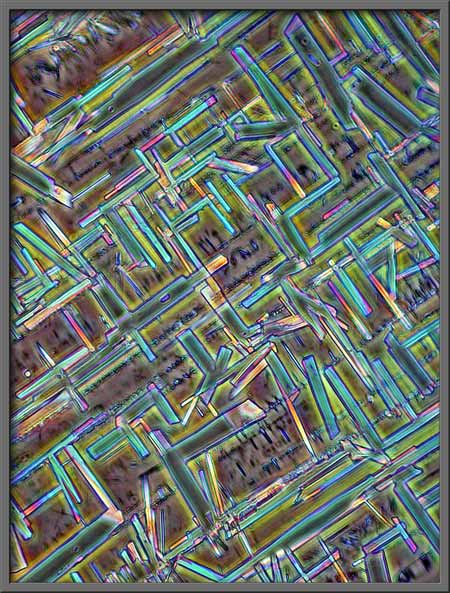
In each of the following pairs of
photomicrographs, the “normal” phase-contrast image is on the left, and
the “inverted” is on the right.


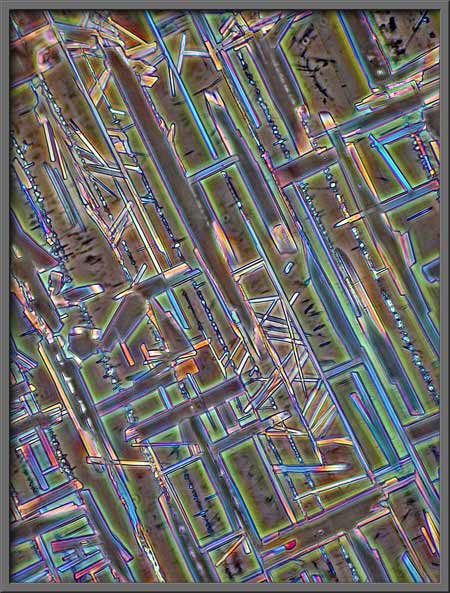

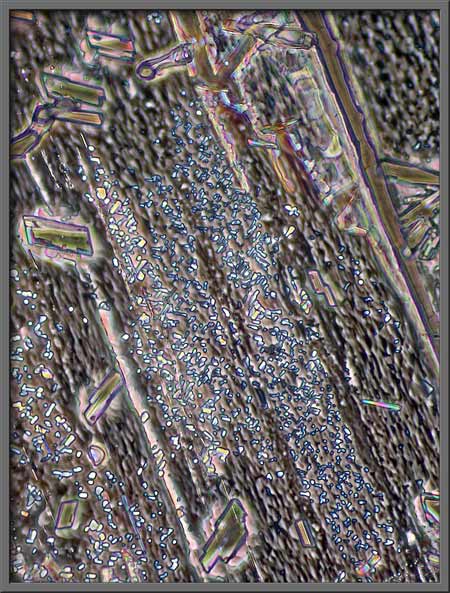



Another post-processing possibility
is the use of the “Desaturation”
tool to remove all colour in the photograph. This of course results in
a black and white image.



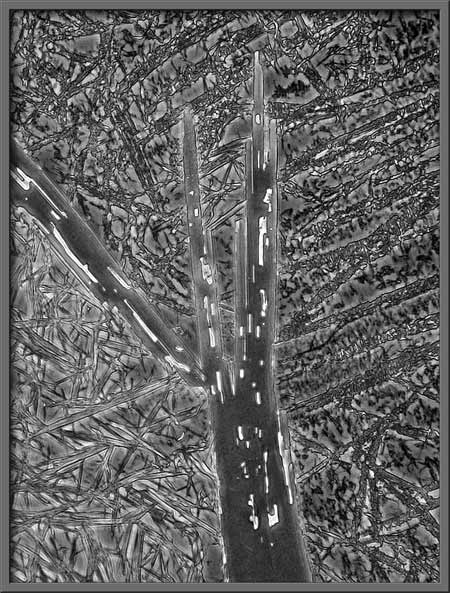
Since many photomicrographers
consider the use of such tools to make dramatic alterations in the
image to be “cheating”, no such changes have been made to the next
group of photographs.
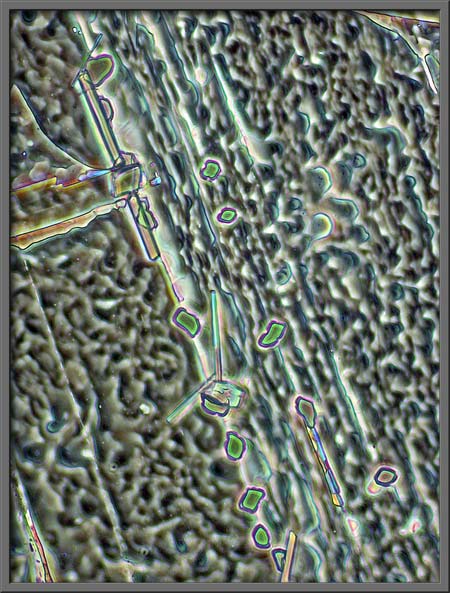
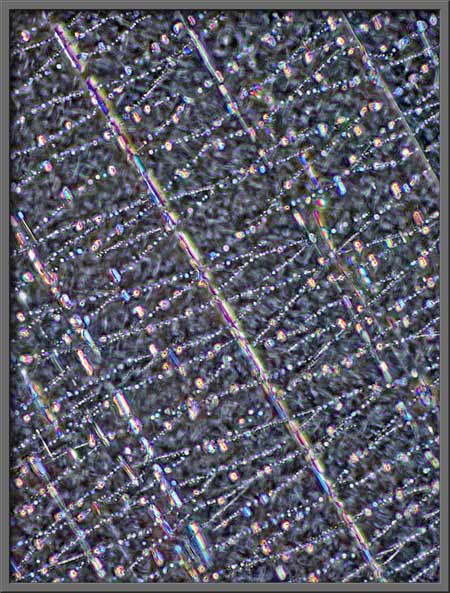
The turquoise rectangles, mostly at
right angles to one another, make a rather artistic arrangement.
Large areas of the field under the
cover-glass are rather amorphous, but even here there are interesting
details. Note the hair-like X’s between the darker parallel lines
in the image on the right.
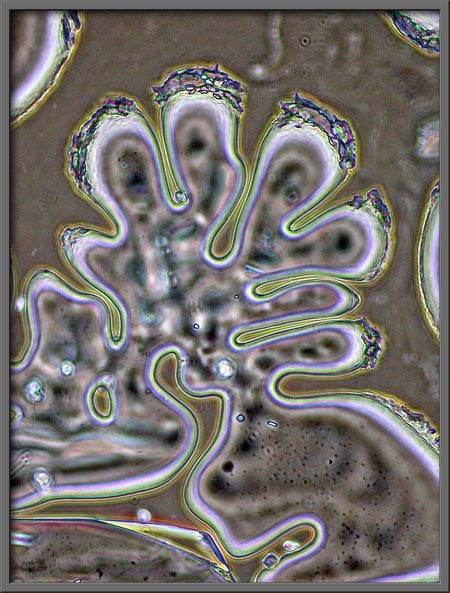
After the slides used in this
article were prepared, the edge of each cover-glass was ringed with a very thin bead of fingernail
polish. The strange lobed pattern below is the result, and was
found at the very edge of the cover-glass. Note the tiny benzoic
acid crystals re-crystallizing from the fingernail polish solvent as it
evaporates.
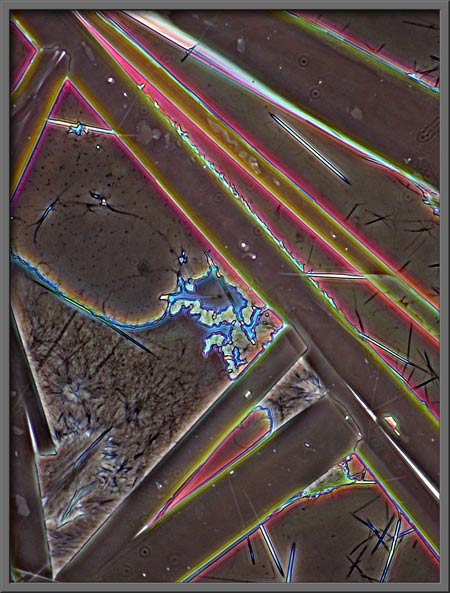
The following seven
photomicrographs show some of the more interesting fields that I
observed.


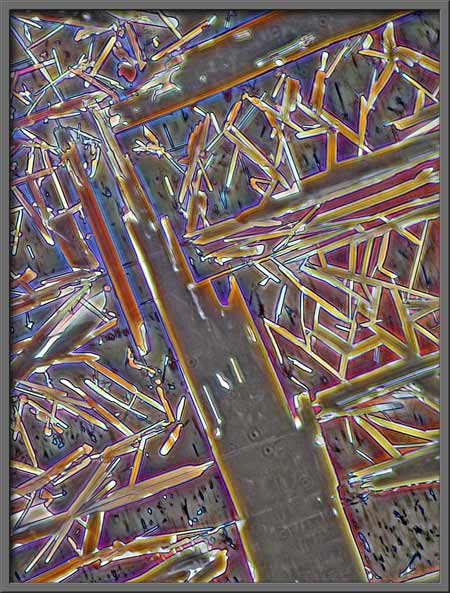


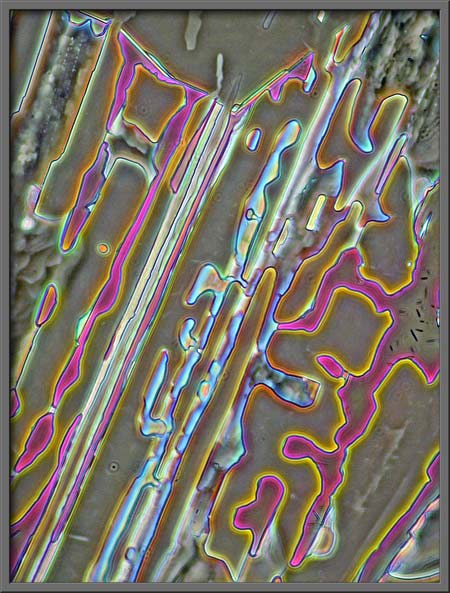
If you have used phase-contrast
illumination on biological specimens, you know that the visual
(apparent) colour saturation of the background is controlled by the
intensity of the light source. In post processing, the use of the
“Levels” tool can mimic this,
and increase the contrast between foreground and background.
The background can be slightly
darker than normal.
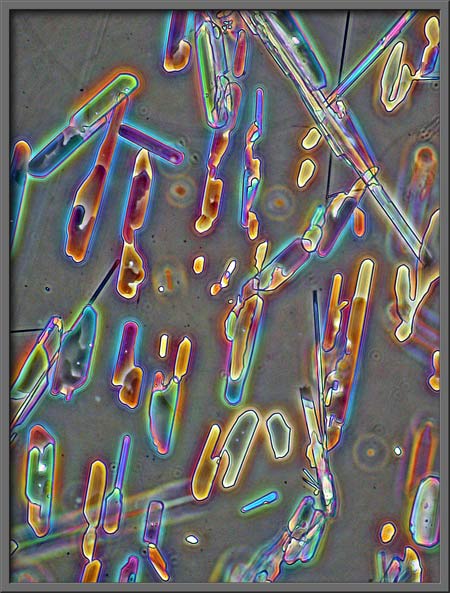
It can be darker still.

It can be almost black as in the
two images below.


Benzoic acid fields may contain
very structured forms,
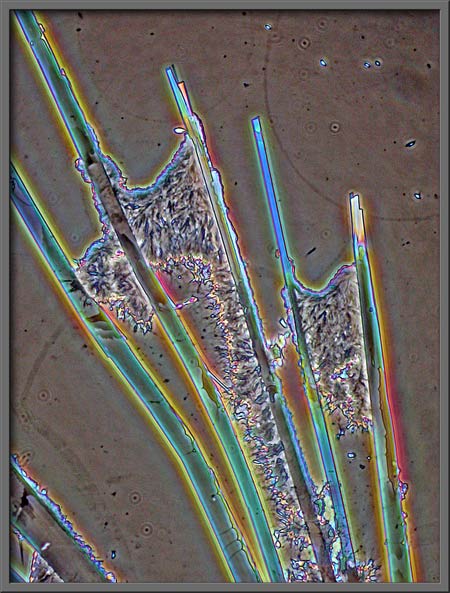
areas with mixtures of structured
and unstructured forms,

and areas which are more random.

The occasional field may even look
like the one below. Even if it is the photomicrographic
equivalent of the pile of plastic excrement mentioned earlier, both
share the same advantage- neither smells!

Published in the
September
2006 edition of Micscape.
Please report any Web problems or
offer general comments to the Micscape
Editor.
Micscape is the on-line monthly magazine
of the Microscopy UK web
site at Microscopy-UK
© Onview.net Ltd, Microscopy-UK, and all contributors 1995 onwards. All rights reserved. Main site is at www.microscopy-uk.org.uk with full mirror at www.microscopy-uk.net .