Who would have thought, more than
a hundred years ago when these molecules were first
studied, that this strange state of matter would become as
critically important as it is today? Most households have
calculators, cell phones, digital cameras and perhaps
computer monitors containing an LCD or liquid crystal
display. The compounds used in its creation are almost a
contradiction in terms. Liquids flow, while crystals are
solid! Liquid crystals are unique, in that they combine
the physical and optical properties of both states of
matter.
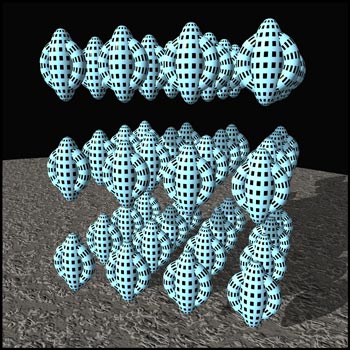
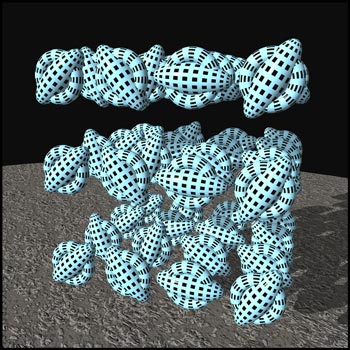
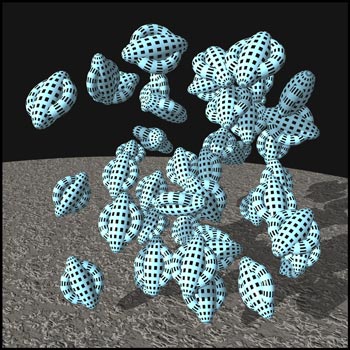
The molecules that make up liquid
crystals are attracted to one another. However, these
intermolecular forces have different strengths in
different directions, because of the tubular shape of the
molecules. At low temperatures, there is little thermal
motion, and all the forces hold the molecules in a rigid
lattice arrangement called a
solid
crystal
. The crystal is composed of many layers of molecules,
some of which are represented in the graphic below.
Notice that the molecules are in definite positions and
are oriented in a definite direction. Thus a crystal has
positional as well as orientational order. (This and the
next two illustrations were produced using the
'Bryce
' ray-tracer.)
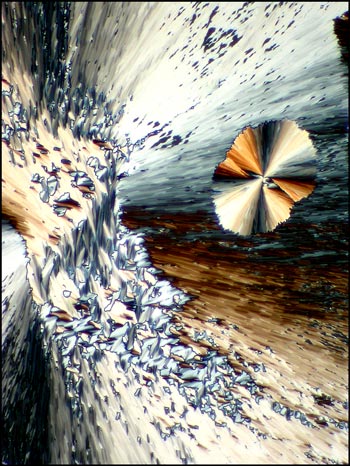
All of the compounds
photographed in this article are referred to as
'thermotropic', because they alter their properties
when the temperature changes. There are many such
compounds available. I have chosen cholesteryl
benzoate (the first liquid crystal to be studied in
1888), cholesteryl stearate, cholesteryl laurate and
three compounds labelled liquid crystal A, B and C.
Unfortunately the last three were obtained twenty-five
years ago, and there is no way of easily determining
their structure.
The compounds are all white
crystalline solids at room temperature, and have
melting temperatures less than 200 degrees Celsius. In
order to prepare a specimen, a very small quantity of
the substance is placed on a slide and covered with a
cover glass. An alcohol lamp is used to melt the
solid. For many liquid crystal compounds it is
important to study and photograph the results quickly.
The liquid crystal state exists for a relatively short
time (minutes) as the compound cools back to the solid
crystal state. Initially, while in the liquid state,
the 'crossed polar' observation results in a black
field. (The liquid is of course isotropic.) As the
liquid crystal state forms, extraordinary crystal
forms are observed. (These are anisotropic.) After a
few minutes, it is fascinating to watch as the solid
crystal state slowly takes hold, resulting in a
completely different crystal structure. (These
crystals are also anisotropic.)
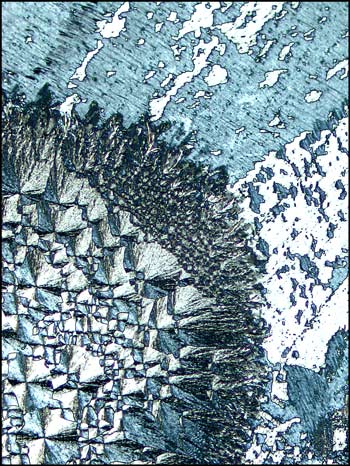
The next four images show the
process of changing from the
liquid crystal
state to the
solid crystal
state. In each, you will observe
circular forms which are growing larger at an alarming
rate as I attempt to photograph the field. (Within
several minutes, each field is entirely covered by
these solid
crystal circles which
eventually grow together, obliterating the
liquid crystal
formations which can be seen
outside of the circles.) The circular solid crystal
formations are maintained until the slide is reheated.
Researchers use specially adapted heating stages on
their microscope to maintain the temperature within
the liquid crystal range.
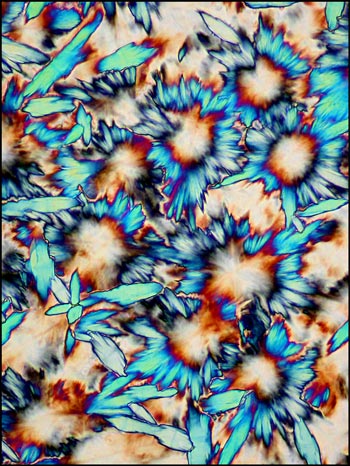
By using higher
magnifications it is possible to observe interesting
detail in the liquid crystal formations. The three
images below show strange circular growths that
frequently form in this phase. (You will have to study
the edge of the last image carefully to see the
beginnings of loops.)
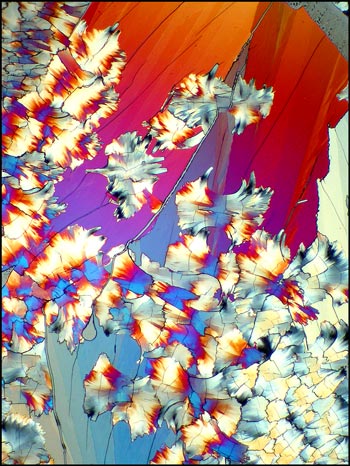
Crossed polars were used in
all photographs, but in some, compensators
(l
/4 and
l) were used to alter the visual appearance of the
crystal forms. Occasionally this results in the field
having an almost three-dimensional
appearance.
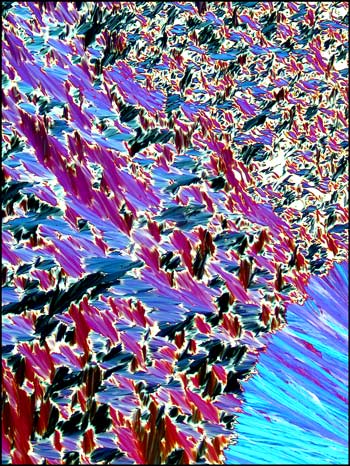
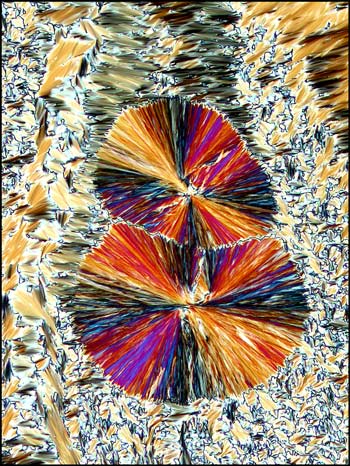
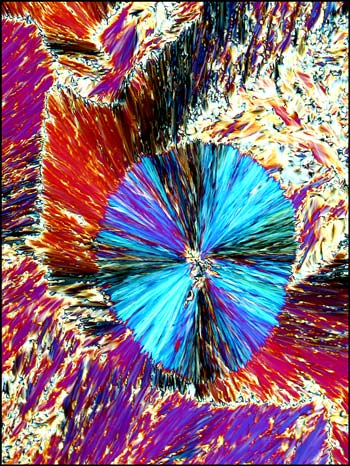
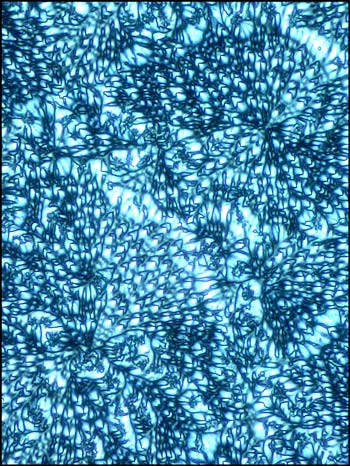
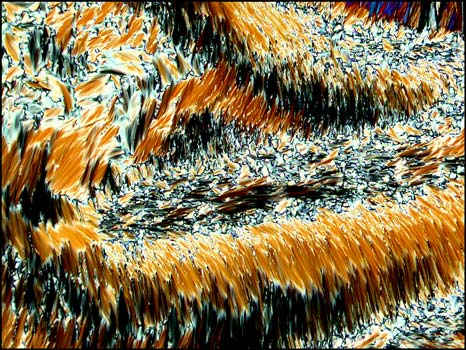
Liquid crystals produce
patterns that are unlike any that I have observed with
other compounds. The following photomicrographs show
the diversity of forms produced by this unusual state
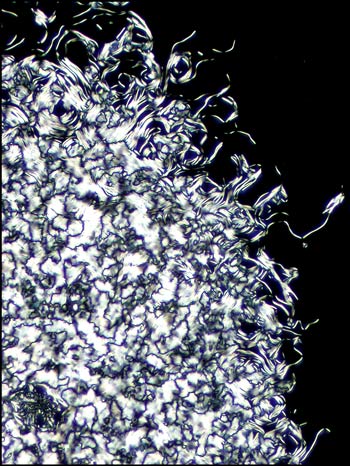
of matter. Although the first is not colourful, it is
one of my favorites. Many distinctive forms keep
showing up as one looks at a multitude of slides, but
this one was truly unique!
A Leitz SM-Pol microscope and
Nikon Coolpix 4500 digital camera were used to take
all of the photomicrographs in the
article.
The low melting points of
most liquid crystals make them easy to study under the
polarizing microscope. I hope that the images shown in
the article prompt you to have a look for yourself.
These compounds are a truly fascinating field of
study!
Note: Liquid crystals are
considered moderately hazardous and should be handled with care. Proper
ventilation is important when heating the substances. Discarded liquid crystal
displays should never be disassembled in order to obtain compounds to
study!
All comments to the
author
Brian Johnston are welcomed.
Link to further
information about liquid crystals:
University of Wisconsin-Madison, 'Science is Fun in
the Lab of Shakhashiri', Liquid Crystals.