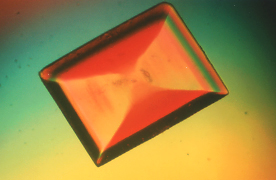
| Looking
at perfectly formed crystals under the microscope is always fascinating.
Ordinary "chemicals" around the home such as salt and sugar can be crystallized
from water solutions on a microscope slide to provide interesting specimens.
Even with random crystallization's that will produce mostly distorted specimens,
some perfectly formed cubes and rectangles from table salt and orthorhombic
crystals from sucrose (sugar) make interesting specimens for viewing. This
study can be continued on other materials from the kitchen and medicine
cabinet—citric acid from unsweetened Kool-Aid, aspirin,
starch grains, etc.—to provide a number of interesting
crystals to view under the microscope with both regular and polarized light.
Although these make interesting specimens for viewing, they are still "ordinary"
crystal specimens. |
|
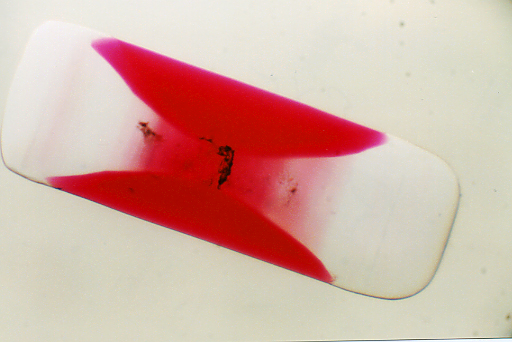
| Occasionally,
however, some unusual crystallization phenomenon is discovered, studied,
and described in the literature that piques the curiosity of chemists,
mineralogists and crystallographers. One such phenomenon is the growth
of hourglass inclusions in the crystal lattice. Hourglass inclusions were
originally observed for various chemical pairs over 150 years ago and were
extensively studied in the 1930s by Buckley in Manchester, England. Out
of over 16,000 combinations of chemical pairs he studied, he was able to
document only a handful of materials that crystallize in this manner. As
one might expect, such unusual crystal growth is quite rare in crystallography.
In the following example, acid fuchsine dye will
grow under favorable conditions in the crystal lattice of potassium sulfate,
not turning the whole crystal red, as one might expect, but forming a red
color only in certain regions of the crystal lattice to form an hourglass
looking structure. (For a more thorough discussion of the crystallography
taking place see reference (1) below.) This is perhaps one of the easiest
to demonstrate and study hourglass crystal structures.
|
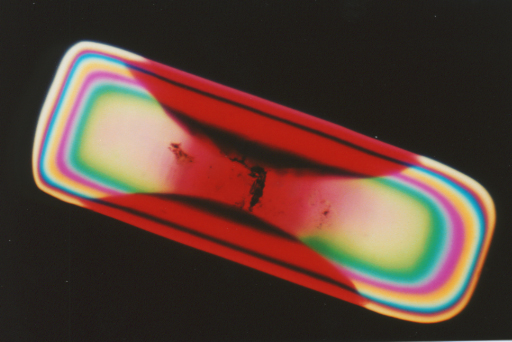
Directions
for preparing micro specimens of these crystals have been taken from the
original reference (1) and adapted for growth on a microscope slide (2).
These crystals are very interesting and unusual specimens to observe under
the microscope in both regular and polarized light.
Procedure:
-
Prepare a 10% solution
of potassium sulfate by dissolving 10 grams of K2SO4
in 90 ml of deionized water. Since this is at, or near saturation, the
solution may have to be heated slightly to get all of the solids dissolved.
-
Prepare an 0.1% solution
of acid fuchsine dye by dissolving approximately 0.1 gram of acid fuchsine
in 100 ml of deionized water. (Acid Fuchsin, sodium salt, Cat. No. 33,270-4,
Aldrich Chemical Co., Milwaukee, WI, 53233, USA)
-
Add 10 drops of 10%
potassium sulfate solution to a microscope slide. Then add 1 drop of 0.1%
acid fuchsine dye solution and mix well.
-
Examine the prep after
about an hour to observe hourglass crystals growing in the mixture.
-
Repeat Steps 3 and
4 if hourglass crystals are not observed. The crystals form only within
certain concentration ranges of these materials and sometimes the drop
sizes are not sufficient to provide these conditions.
-
Isolate and dry individual
crystals for study under the microscope. These can be permanently stored
in dry mount preps (3).
|
| For
those microscopists who do not have access to the chemicals mentioned above
but would still like to observe these crystals firsthand, you can contact
me for a slide prep of some of these crystals. I will trade you for an
interesting slide prep of your own or send you a slide for a small fee
to cover the cost of a slide mailer and postage.
You may contact me by e-mail Jim
Benko.
References:-
1. Bart
Kahr, Jason Chow, and Matthew Peterson, Organic Hourglass Inclusions—A
Review of Past and Recent Work and a Student Experiment, Journal of Chemical
Education, Volume 71, Number 7, July 1994, pp. 584-586.
2. James Benko, Letters to the Editor, Organic
Hourglass Inclusions, Journal of Chemical Education, Volume 72, Number
10, October 1995, p. 956.
3. James Benko, Micscape
Practical Tip: A Quick and Easy Way to Make Drymounts,
Micscape Magazine, July, 2000
Top
of Page
|
|
©
James Benko & Micscape Magazine - Sep 9th 2000
|
|