|
Topical Tips: The multipurpose micrometer slide—much more than just for calibration.
by David Walker,
UK
|
Most microscopy enthusiasts are likely to have at least one micrometer slide in their accessory box as reliable measurements are often required for many studies. In addition to its primary role of calibration it has a variety of other roles and examples are illustrated below. High quality multi-scale slides are readily available now for less than £10.
|
Right: Typical examples of the styles of slide available from my own collection, comments on their features are below.
1 - A typical older 19th century micrometer slide often found in purchased slide collections. This example is made of compressed card rather than glass. Engraved lines of different spacings were typical at the time rather than a numbered scale. This example has vertical lines spaced at both 100 and 1000 to the inch.
Although calibrating in inches is very dated, this form still has value as the spaces are 25.4 and 2.54 microns. The finer spacing is a quarter of the typical modern 0.01 mm (10 micron) scale so useful at higher magnifications.
(I don't recognise what looks like the maker's ornate monogram on this slide, comments welcomed.)
Note added Oct. 15th 2015. Thank you to Howard Lynk who notes that the slide "... was made by Smith and Beck to accompany their less expensive models. ... They also made all glass ones to accompany their higher end models." Howard maintains a splendid website on early slides and microscopy - A
Cabinet
of
Curiosities.
The remaining slides all use the modern 'chromed' scales which are very crisp when viewed under the microscope.
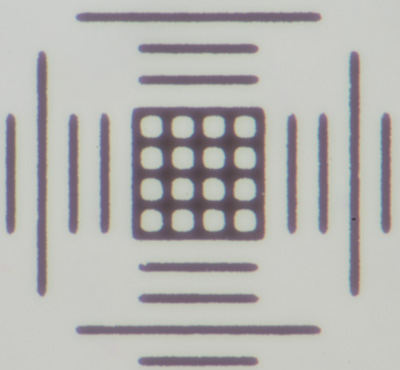
2 - Motic calibration slide. This is a typical form as supplied with dedicated microscope cameras in this case with a Moticam 1000 for calibrating the capture software. Spots of varying diameter are common plus a central 0.01 mm cross scale with grid square.
The lack of a 1 cm scale of 0.1 mm makes it less suited for the lowest power work.
3 - Typical modern third party calibration slide. This is readily purchased on e.g. eBay direct from Asia for less than £10 including postage and typically supplied in a robust case with shaped foam formers. This is the style I use most as the 1 cm scale of 0.1 mm, the central cross scale / grid of 0.01 mm and spots cover most uses from the lowest to highest powers.
4 - Single 0.1 mm scale calibration slide. These are usually the cheapest but are the least versatile. A 0.1 mm scale alone is too coarse to accurately calibrate higher powers, the lack of a cross also makes it less suited for vibration checks in photomicrography as described below.
This example does have a coverslip. Slides 2 and 3 are typically uncovered but for objectives designed for a coverslip, a temporary slip can be used with water as mountant.
|

|
Primary use for calibration. For readers unfamiliar with its primary use, the Micscape Library has a selection of articles (type 'micrometer' into the 'Find' page on top menu). Examples of uses include:
- Calibration of an eyepiece reticle for each objective mag. Ideally an eyepiece with a reticle permanently mounted is most convenient to keep the scales scrupulously clean as any dirt will be in the plane of focus. I don't have a spare suitable eyepiece for either my compound or stereo scopes and prefer not to have a reticle permanently in view. So this measurement method is one I rarely use.
- Calibration of a filar eyepiece which are preferred by some users to a reticle, but they can be expensive.
- Calibration of a dedicated microscope camera's software where it includes a measurement feature.
- Calibration of the horizontal field width HFW of a camera's field of view when mounted on a microscope for each objective or stereo mag setting. This is my preferred method of measurement as I use a consumer camera rather than a dedicated microscope camera. A feature of an unknown subject is measured in pixels on the screen or my preference is to print out and use the HFW in conjunction with a ruler. As this method is effectively calibrating pixels it could be regarded as a manual form of the
approach that capture software uses. Declaring the HFW of a presented image is also an alternative to adding a scale bar and for some subjects the HFW can be arguably of more value to a viewer than a scale bar for them to judge size of a gross object.
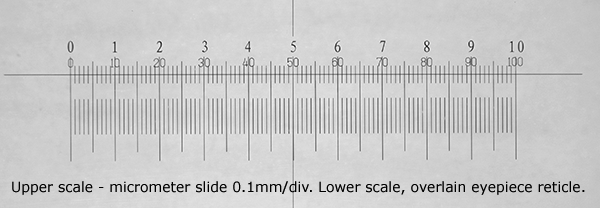
Above. Calibrating the eyepiece reticle in a stereo microscope with a micrometer
slide. The typical continuously variable zoom optics of a modern stereo can be
adjusted while viewing to match the two scales exactly for easier reading off of
values. Although unlike the fixed mag of a compound microscope objective, the
user needs to be careful that exactly the same zoom position is set if not
click-stopped. See Micscape April 2013 article.

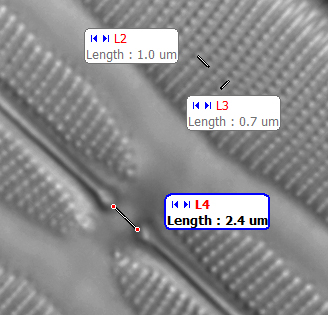
Above. Once calibrated, the typical dedicated microscope camera's software can readily perform a range of measurements and derived calculations i.e. linear, areas, angles and also counting. This example using a Moticam 1000. See Micscape March 2005 review of this type of camera. Third party image capture freeware such as Marien van Westen's versatile Micam
and Dicam (the latter a neat variant of Micam for consumer DSLRs with Live View) has similar features.
Aligning a flat subject under a Greenough stereo for photomicrography. A stereo is useful for photography of larger subjects unsuited for the compound. If using a Greenough rather than a Common Main Objective design, the former will give a poor image for a flat subject spanning most of the field of view because its angled optics will not retain focus across the field. This is easy to remedy by angling the subject support
at ca. 6° (half the typical subtended angle of the optics) so that it is presented at right angles to the optical axis. Trigonometry can readily calculate the height of the support required at one end. To check that the correct angle has been set, the micrometer slide can be used to check in focus across the field, as well as calibrating eyepiece reticles for measurements at the same time. At lower mags, as example below, the 1 cm scale may not span the field but can just check half the field by aligning
one end at field centre.

Above. Micrometer slide flat on stage. 1x zoom on Meiji EMZ-1 stereo, half field, '100' focussed near field centre. Loss of focus from the centre edge.
This is the best case at the lowest mag, the effect will become more pronounced as the mag is increased and depth of field decreases. (If a long thin linear subject, the effect can be minimised by aligning vertically if don't wish to angle the subject.)

Above. Same set-up but with slide supported 7 mm at lefthand end. Focus is now even across field. The improvement is less obvious at 1x zoom as depth of field may partly correct.
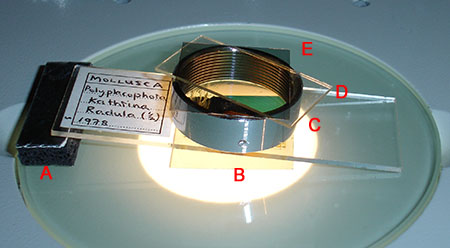
Above. A slide supported one end for stereo photomicrography under a
Greenough stereo. For a 3 x 1 inch slide as used here, the support required is
ca. 7 mm). In this case cross polarisation filters with retarder are also being
used, raising the analyser/retarder on a collar keeps them out of the plane of
focus. The polariser is also out of focus. See November 2008 Micscape
article.
Using a micrometer slide cross-scale for checking vibration on a microscope-consumer camera set-up, especially if camera hard-coupled. Some modern mirrorless digicams can be the worst vibration offenders.
It is good practice to check any microscope-consumer camera set-up for vibration at all the likely mags it will be used for. This especially applies if the camera is hard coupled rather than independently supported from the microscope on a stand, although the latter set-up can introduce additional potential degrees of vibration if not a very sturdy stand. Gross vibration can be readily detected with any suitable subject, but residual vibration can be subtle and if present may just take the
edge of potentially sharper imagery.
A micrometer slide, especially the modern examples with chrome scales, can be useful for checking vibration which will be evident as a loss of crisp scale edges. A cross-scale is more useful in this regard as can check vibration that may be solely in one plane. From my own experiences with mirrorless digital cameras, they can be particularly prone to vibration—although the potential vibration of a mirror is removed,
the exposed sensor cocks its mechanical shutter right at the beginning of the exposure in addition to the end.
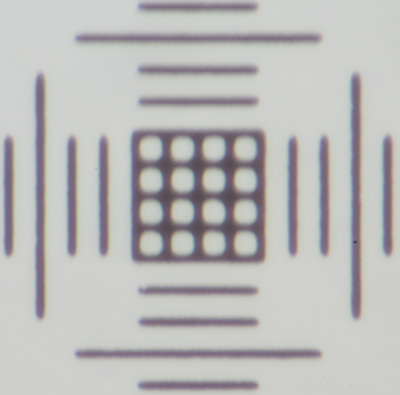
Above. Sony NEX-5N APS mirrorless body hard coupled to a Zeiss Photomicroscope III, 0.01 mm cross scale of micrometer. Great care taken with use of shims to ensure absolutely no flexing in the scope-camera coupling in all planes even when flexed with force.
Left: Objective 16X, Optovar 2X, electronic first curtain shutter (EFCS) off, 1/125th sec. At this modest objective mag there is already image softening, the cross-scale showing that it's primarily on the y axis, the same as the shutter travel.
Right: Same set-up and shutter speed but with EFCS on. Similar vibration problems were observed if EFCS not used even on a stereo. I don't use a mirrorless camera hard coupled to a microscope unless it has EFCS. My brother had similar vibration problems with a Panasonic GF2 mirrorless camera.
On my previous Nikon budget reflex mirror DSLRs without mirror lock, but 1 sec shutter delay, I could use any shutter speed for the 16X objective and below on the same camera couplings. See Micscape November 2012 article for extended discussion and examples.
Assessing optical performance of objective-eyepiece, camera-relay lens etc.
The opaque lines of a micrometer scale with crisp edges against a bright background in brightfield are a demanding subject for the microscope. It is a useful subject for assessing the complete optics train of objective-eyepiece plus relay lens for photography and can often reveal any problems, either visually or with camera. These include assessing:
- planarity across the field
- lateral chromatic aberration, especially towards field edge—may indicate e.g. deficiencies in objective, an objective-eyepiece mismatch, not ideal relay lens
- geometric distortion e.g. barrel distortion towards the edges
- the horizontal field width of a relay lens with camera cf the visual field
Ideally a maker's recommended eyepiece should be used with their objectives to minimise aberrations but there may be occasions where a mismatch is preferred. The ideal relay lens for photography can be eagerly sought after and often reflected in the high prices they fetch, so a user may opt for a relay lens such as a visual eyepiece with collar to project a real image.
Camera lenses are also potentially useful as relay lenses in so-called afocal mode but can vary in their suitability. The micrometer slide can check if there are any mismatches and if so, their severity.
On my Zeiss Photomicroscope III, I use the Zeiss 8X or 10X KpL visual eyepiece in specific modes as a projection lens for APS sensor digital cameras. A micrometer slide shows whether a good match at the image sizes likely to be used. When assessing an image of a micrometer
slide e.g. for chromatic aberration, some zooming in is useful but it's best to beware of pixel peeping. The current very high resolution sensors may show some CA approaching 1:1 but this is looking at a poster sized printed image. The results may be quite acceptable for screen use and modest sized prints.
The micrometer slide also indicates the field of view covered by a camera sensor cf the visual field. How much cropping is acceptable may be a personal preference and on the type of subjects studied. Some cropping of the visual field can be useful for many subjects e.g. small pond life—in the same way that wildlife photographers
often prefer the APS sensor to
full frame as there's a boost in the lens focal length because of the sensor cropping effect.
The example below shows that a Zeiss 8X Kpl eyepiece was poor for direct projection without camera lens but excellent in afocal mode with a lens on the camera—both in control of aberrations and a field of view near optimal.

Direct projection: A Zeiss 8X Kpl eyepiece on small collar to give a real image for direct projection into a Canon 600D DSLR body. Full sensor field. Marked CA is present and also asymmetric.
Afocal: The same Zeiss 8X Kpl eyepiece, no collar, projecting into the Canon 600D with a Nikkor 50 mm f2 lens attached. No evidence of CA (in fact almost absent if pixel peep) and captures the same field of view as the built-in 35 mm film camera in the Zeiss Photomicroscope. Also little geometric distortion and good planarity. A < £10 52 mm afocal microscope adapter was used to couple the front of the camera lens to the eyepiece. See Micscape September 2013 article.
Using the 0.01 mm scale to assess the optical performance of a stereo microscope (or attached camera set-up) at the 1X mag.
The numerical aperture of a stereo microscope is low at its typical native 1X optical mag—good quality optics can only just resolve a micrometer slide with a 0.01 mm scale which requires an NA of 0.0275. A micrometer slide is thus a useful test object. The scale occupies a small part of the field with the typical standard 10X eyepieces and is just resolvable to the eye with good visual acuity and also serves as a test for a stereo-camera combination. A well matched camera set-up should also be
able to resolve it if the optics can.
This test also illustrates a feature of zoom stereos that the makers don't often declare—they usually give a single point NA value at its maximum magnification rather than the NA across the range. The numerical aperture at 1X of a modest range zoom stereo of e.g. 1-3X is superior to that at 1X of wider range stereo e.g. 1-8X even if the latter has higher quality optics. So if most stereo work of
interest was at the lower mag end and resolution was key, there is no optical benefit of buying a wider range stereo. This is illustrated below with two Greenough stereos—a Leica S8 APO 1-8X fully apochromatic and a Meji EMZ-1 1-3X achromatic. At 1X the Meiji resolves the scale whereas the Leica at 1X does not. See Micscape May 2013 article for an extended discussion and illustrations, where NAs are measured and plotted with mag settings.
A maker's flagship macroscopes could fail this test at 1X after inspecting typical NA values across the zoom range in their data sheets as they often extend the mag range to 1-8X or beyond. If specialising in high resolution macrophotography, rather than use a stereo, many users adopt a long working distance fully corrected compound microscope objective with suitable relay lens and extensions—often
coupled with stacking to give striking results. These optics can have double the NA at a low mag of the finest stereo or macroscope.


Above: A 0.01 mm micrometer slide, unmodified image except resized crop from master using 1X optical setting on both stereos.
Left - The Leica S8 APO 1-8X and right, the Meiji EMZ1 1-3X . (Electronic first curtain shutter mode if a Sony NEX 5N body to ensure no vibration.)
Resolution may not be a key feature with stereo use. Contrast and colour rendition may be factors. The APO gives cleaner rendition of a neutral colour than the Meiji.
The author David Walker welcomes any comments.
©
Microscopy UK or their contributors.
Published
in the October 2015 edition of Micscape.
Please
report any Web problems or offer general comments to the
Micscape
Editor .
Micscape
is the on-line monthly magazine of the Microscopy UK web site at
Microscopy-UK
© Onview.net
Ltd, Microscopy-UK, and all contributors 1995 onwards. All rights
reserved.
Main site is at www.microscopy-uk.org.uk.












