The
inner epidermis of the onion bulb cataphylls
(the
onion skin)
Easy
and not so easy methods to work with
Walter Dioni
- Cancún, México
Still images of cytosol
streaming in epidermis of onion cell
Continued
from part 6 – Fixing with Clarke’s fixative - Staining with Blue 1, and Eosin
I’m well aware that all samples of onion skin that could
be taken will show the same arrangement of polygonal cells, with a cellulosic
wall, cytoplasm, vacuoles and nuclei. In this series I have
given extensive testimony of this, and virtually all of the images (whether
drawings or pictures) that occur on the Internet show the same.
http://www.sciencephoto.com/image/10537/530wm/B0600062-Cytoplasmic_streaming_in_onion_cells-SPL.jpg
That’s all. Only one snapshot of the
dynamic features of an onion cell.
http://www.youtube.com/watch?v=wkbijKyM4eQ (Riveal
contrast)**
http://www.youtube.com/watch?v=VXbQpRpUDmQ&NR=1*
http://www.youtube.com/watch?v=cLgHnJEmoI0
|
* The flower is
less than 1.5 cm in diameter. Preferably under a stereoscope should be cut
with fine-tipped tweezers one or more of the hairs at the base of the stamens,
mount them in distilled water. Cover them and see, through the 100xOI, the
cells that compose it. If the temperature of the preparation is good and the
flower was in active development, there is a great chance of observing this
beautiful phenomenon. |
I do not know pictures of preparations fixed and coloured, showing stills from the
streaming in onion cell.
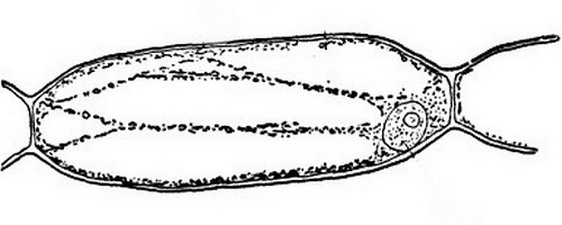
And
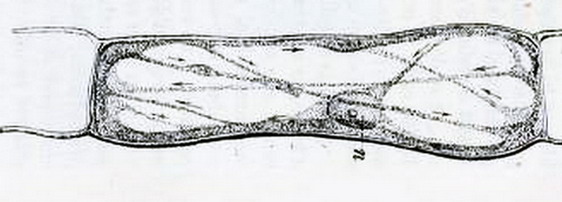
also this better one, of the same time, from a Chelidonium (fig 2)
A modern image of the onion cell structure, that
synthesizes the knowledge gathered through many years, and with the use of many
techniques, including TEM, the live observations, and biochemical analysis is
the following:
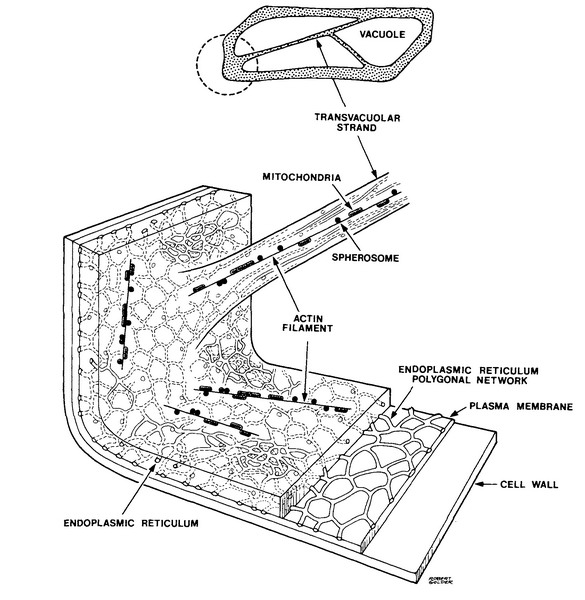
Fig
3.- (Source:
N. S. Allen and D. T. Brown, 1988. Dynamics of the Endoplasmic Reticulum in
living onion epidermal cells in relation to microtubules, microfilaments, and
intracellular particle movement. Cell
Motility and the Cytoskeleton 10:153-163 – Wiley-Liss Inc.)
taken from http://advancedlab.org/mediawiki/index.php/Brownian_Motion_in_Cells
Did you recognize the polygonal Endoplasmic
Reticulum shown by eosin in Clarke’s fixed cells in the previous article, and
the trans-vacuolar strands also shown by eosin, and also by iodine in the first
article of this series?
COMBINED
FIXING AND STAINING USING “Acetified Lugol”
I decided to try the Lugol
fixative, acidified with acetic** because its formula makes it a fixative
and a dye at the same time. It incorporates acetic acid ... normally deemed a
good nuclear fixative!, and iodine, which as we saw in the first article of
this series fixes the cytoplasm, and colour of both nucleus and cytoplasm.
|
( **In my articles No Formalin, No Mercury, New Fixatives – Parts 1 and 2,
I named this as Rhode’s Fixative, following a citation in an old edition of Ward
& Whipple’s “Fresh Water Biology”. It is a FOURFOLD
version of the original Lugol’s formulation PLUS acetic acid. So is
different enough from the Lugol* to merit a new name. But I have not found
any reference to the original publication, nor to the formula’s author, and
in all publications on plankton techniques I see, the formula is called
Acetic Lugol, or worse, only Lugol. So I feel that I must accept the
fashion's trend ... ) *( LUGOL – It was mixed for the first time
in 1829, and named in honour of the French doctor J.G.A. Lugol – (Wikipedia) |
Potassium Iodide 10 g
Water 100
ml
Iodine 5 g
Acetic acid 10 ml
Dissolve the KI in the warm water. Add the Iodine and
dissolve completely. Do not reverse this order. Incorporate the acetic acid.
This formula is accepted as a very good fixative for
plankton samples**. Stains and impregnate the organisms, and being iodine, a heavy metal
(with a big molecular weight) facilitates the sedimentation and concentration of
the stained organisms in the sample.
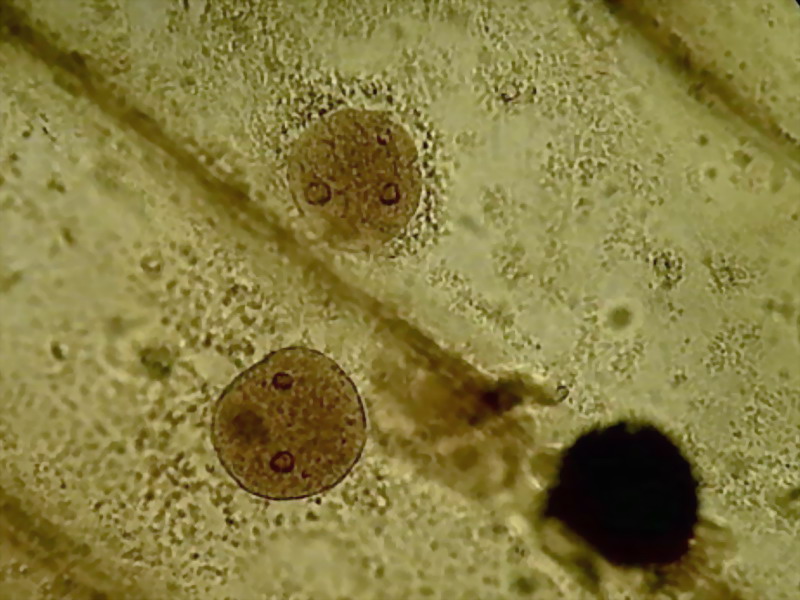
Fig. 4 - Contrast
and intensity were severely diminished to make this picture acceptable
My expectations
were fulfilled.
Setting aside the
strong yellow colour, the cytoplasm images produced by the Acetified Lugol
are, still now, the nearest to the live image, and even better than the ones
recorded using the iodine tincture in the first part of this series, which I
rated high. Not so the nuclear images. They are displayed as plump and dark discs,
with none of the typical surface grooves the nucleii have. The grooves are
a real features of nuclei. Almost all of the fixatives reported in the
previous articles show this trait. (And see http://www.plantcell.org/content/12/12/2425.full)
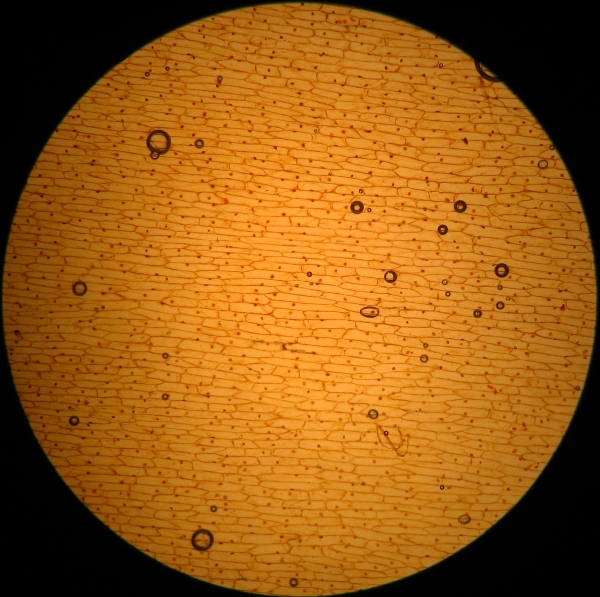
Fig. 5 - 4x total, Acetic-Lugol/10
I had to reduce a lot the concentration of the reagent to
obtain acceptable results.
I made a test with a tenfold dilution:
Potassium Iodide 1
Water 100
Iodine 0.5
Acetic acid 1
There is no reason for a pharmacist not to produce this formula. No
dangerous ingredients, not very expensive also. It is even an aqueous solution.
I also think that this formula merits a name of its
own. I propose Acetic-Lugol/10
Fixation and staining were immediate. No other technique used so far produces a faster
fixing, and a better preservation of which we know by the above videos is the
living structure of the cytoplasm. Many well bounded cytosol strands are extended without distortion, full
of granules, which not being coloured blue with iodine, could be leucoplasts (plastids
with no starch) or mitochondria (people says that mitochondria are destroyed by
acetic acid, but we have here a low concentration...) or Golgi bodies (plant
cells has many of this, but are also a difficult target), ... or simply sphaerosomes
or “vesicles”, a collective name applied by many writers for not to presume the
nature of the granules.
With careful study, the refractive and dark granules allows the easy identification
of the parietal layer of cytosol, and their accumulation around the nucleus,
and in the angles of the cells. Figs.. 5-10
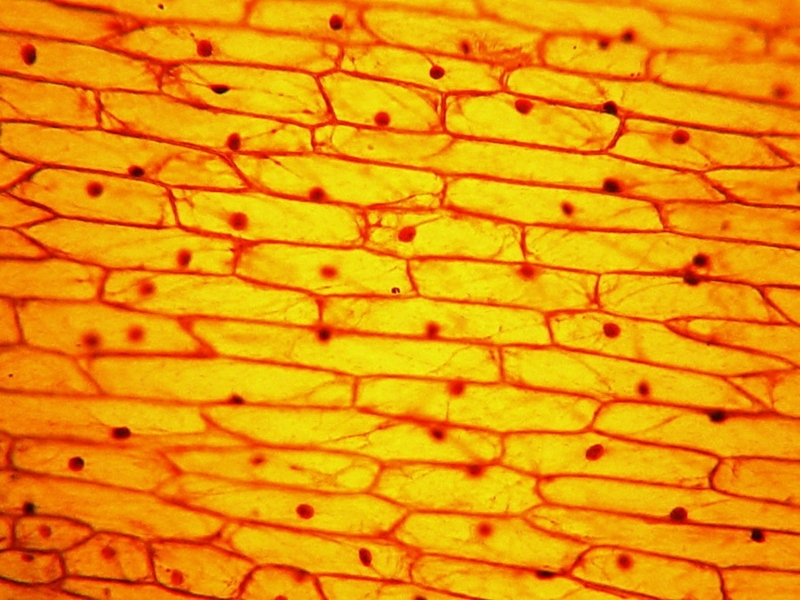
Fig. 6 – Acetic-Lugol/10, 10x obj. See the numerous cytoplasmic
strands even at this low magnification
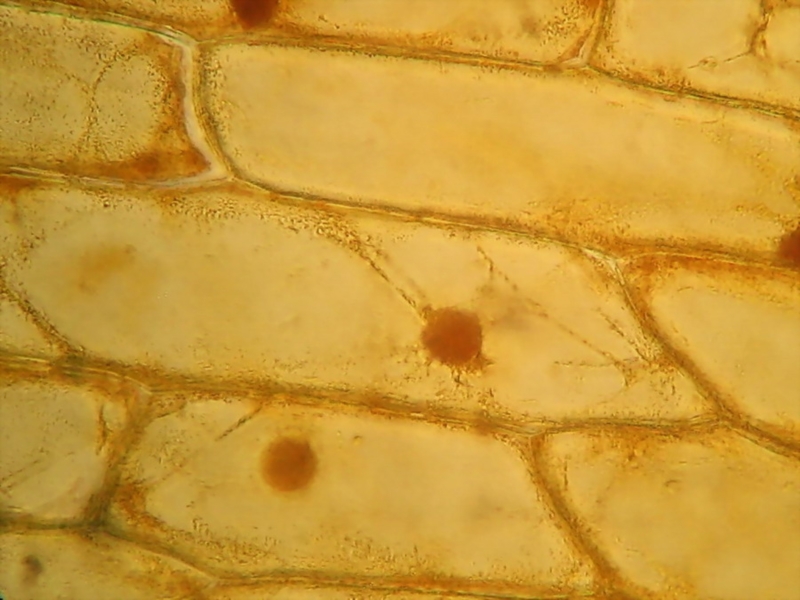
Fig. 7 - CombineZP – Acetic-Lugol/10, 40x obj., 3
images stacked
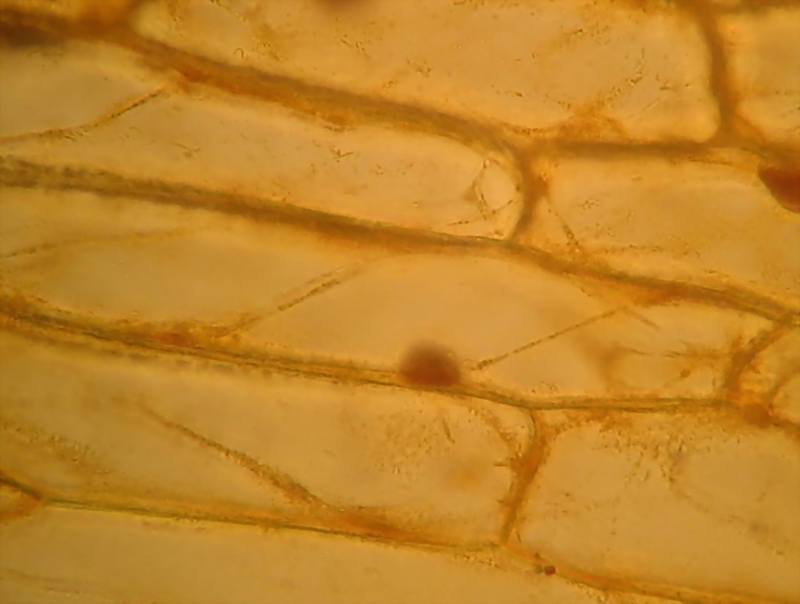
Fig. 8 – Acetic-Lugol/10, 40x obj., 3 images stacked in
CombineZP
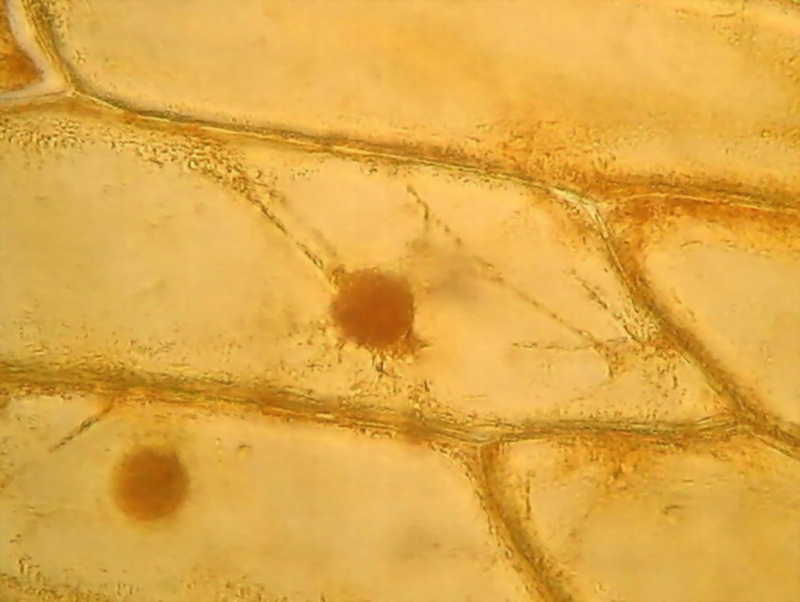
Fig. 9 – This is a detail of picture 7. See the easily
identified delicate cytosol strips, the granulations, and the evident cytosol
parietal layer.
Nuclei are as usual, in various shapes, from oval to strictly circular,
well coloured, granular, with very distinct nucleoli, something darker and refractive.
Nucleoli are the place of formation of ribosomal RNA, and, according to
some graphic representations, it is rolled as a dense ball of filaments. A
careful visual observation at 1000x shows that they seem wrapped in a membrane,
some with one or more portions condensed inside, and others with what is
clearly a central clear point (is it a vacuole?) (not to be confused with a
refraction glare which depends on the focusing). It is interesting that these nucleolar
details can be identified at only 1000x because its description was made from
Transmission Electronic Microscope images.
See http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2107641/pdf/633.pdf, (fig. 13)
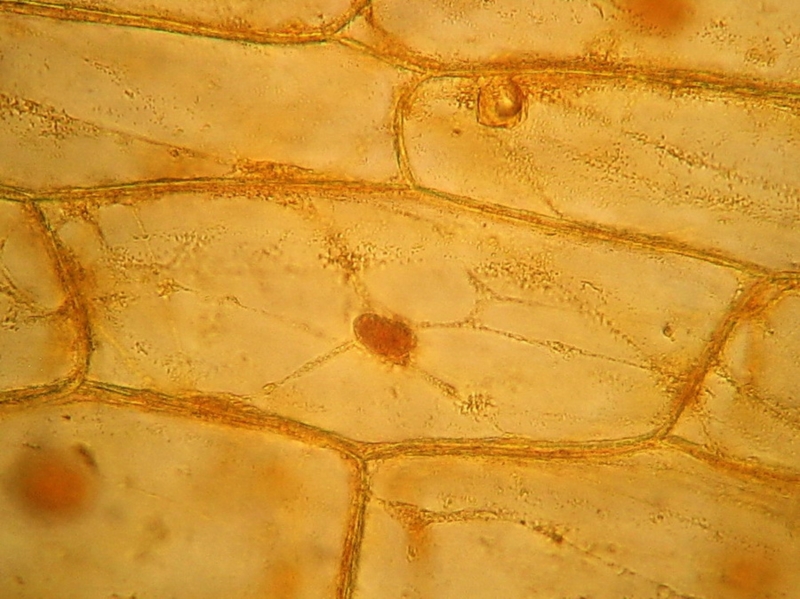
Fig. 10 – 40x obj.,
Acetic-Lugol/10, 4 images stacked in CombineZP.
Canon A75. Handheld camera
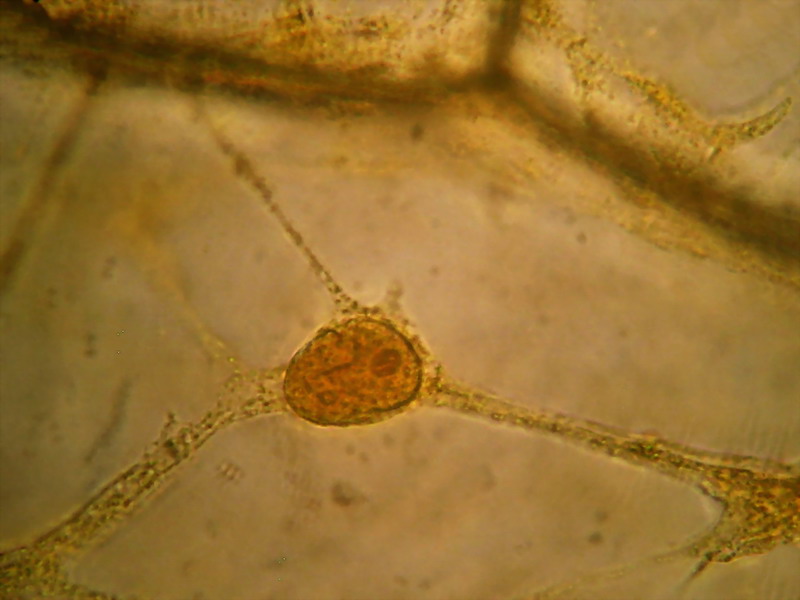
Fig. 11 - 100xOI obj – 3 images, combined with
CombineZP and cropped from a 3Mpx image. Please! Remember that camera (Canon
Powershot A75 in this case) was handheld.
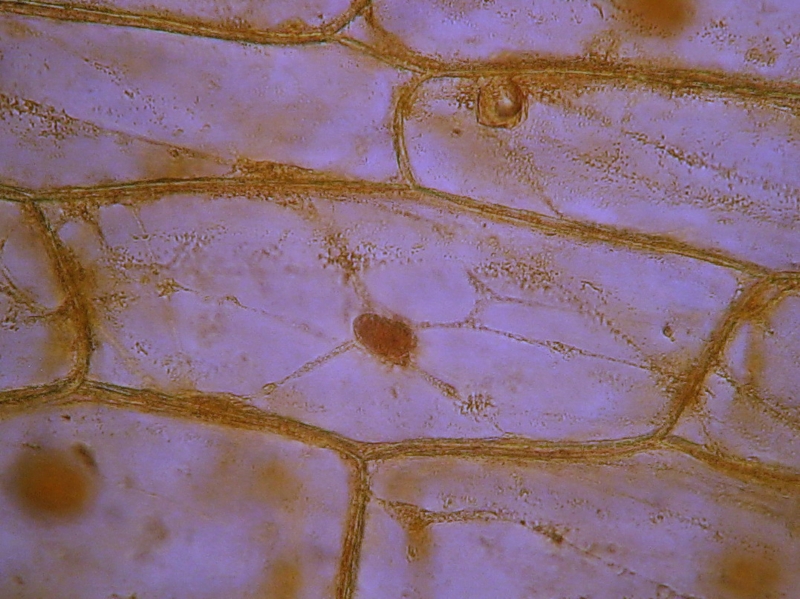
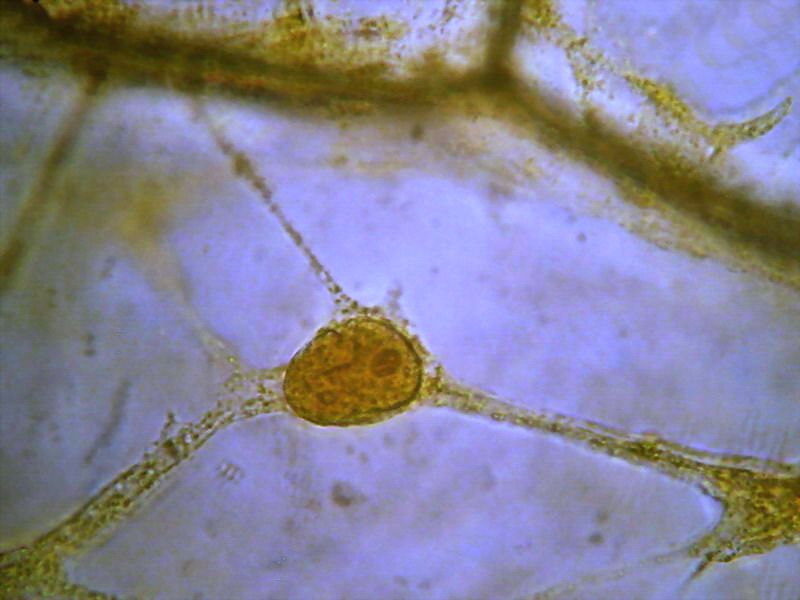
Figs. 12 and 13 – Acetic-Lugol/10, yellow background
eliminated with ACDSee
“tradescantia”
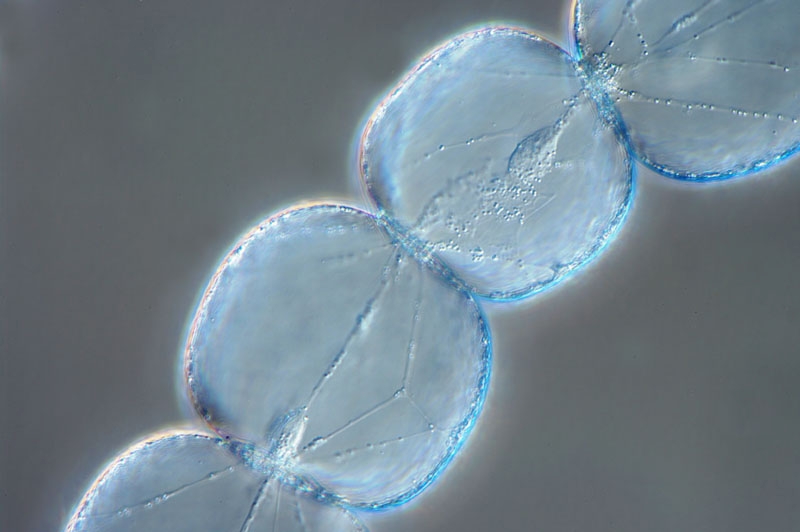
http://www.photomacrography.net/forum/viewtopic.php?p=46295&highlight=tradescantia#46295
Fig. 14 - Many thanks Franz for
the beautiful image and kind permission
Compare with the above images (fig..6 to
13) and the drawings of figs. 1 and 2
Therefore my preparations, fixed and coloured with the
fast ACETIFIED LUGOL, really correspond to the freezing, as a still image, of an
instant in the dynamics of the living onion cell.
Some days after, I had the rare (at least for me)
opportunity, that still now I can not repeat at will, of recording this video which showed me the magnificence of the live streaming. It was an impressive experience.
Streaming in an onion cell
(Editor's note: The above is a YouTube version for maximum cross platform compatibility. The author's master video is a larger video box, of higher quality and can be downloaded here for offline viewing and best viewed full screen, 13 Mbyte avi file. Use the right mouse button to click blue video link and save file locally.)
Since that day my wife is reluctant to use onions in
ours meals. They are alive, she says, refusing to throw them in the boiling
oil! And I couldn’t teach her any technique to euthanize them!
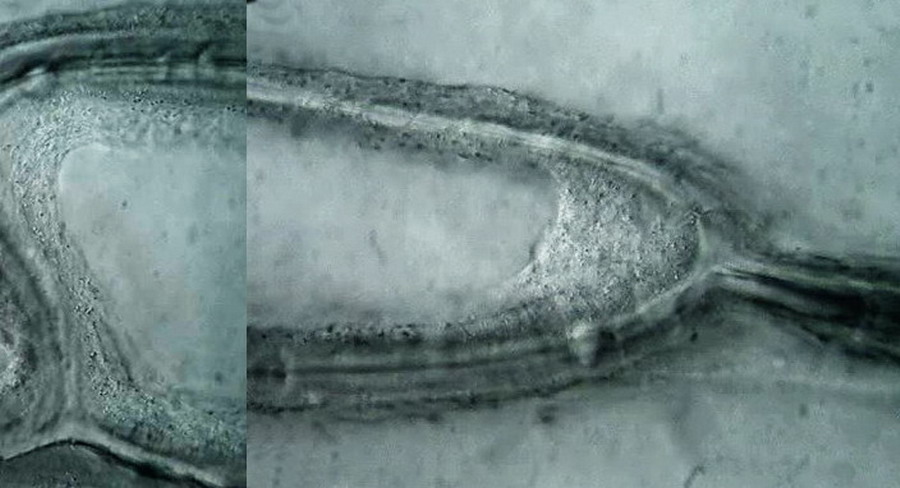
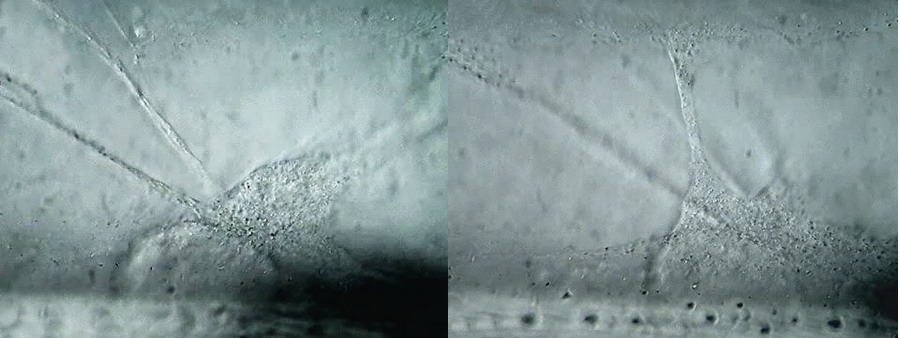
Fig 15 (a,b,c,d) 100xOI obj. Oil inmersión, Circular Oblique Lighting. Logitech Quick Cam Pro 9000. HD-960x720 video configuration – BW. Selected images from the video
Acetic-Lugol/10 could be, then, a fixative of choice
for those that want to show with excellent detail, at 100x, 400x, or 1000x, the
three-dimensional structure of an onion epidermic cell. Pity! ...The 4x
objective image will be inevitably reveal many bubbles.
I hope that any amateur with a compound microscope equipped
with a condenser and a diaphragm, capable of 400x, or better 1000x
magnification, could see the live streaming using only a simple oblique light stop,
or a circular oblique light stop, in the filter holder of his microscope.
Please, take your time! Make a fine adjustment of your lighting before to
start your quest. And be conscious that not many epidermis are in the mood. See this video that show an uncollaborative
cell
http://www.youtube.com/watch?v=0pBQU08kVcg
it shows only Brownian movement of the “vesicles”, no
streaming.
Which could be the clues for the good behaviour of the Acetic Lugol’s,
diluted to 1/10 of its original concentration?
The high concentration of iodine in the original
formula is clearly responsible for the dark staining of nuclei. The diluted solution is feebler and gives
a better legible image. But, the iodine, itself, as a pharmaceutical
tincture, and even diluted, gives not a so faithful representation of
our “live patron” as the diluted Acetic Lugol did (see the first article of
the series).
A high concentration of acetic (in the undiluted Acetic Lugol it is 10%) has
shown in the former trials that it causes a dense precipitation of the cytoplasm
very different from the live image, and that it destroys mitochondria and most
“vesicles”. And even reduced to one percent, like it is in this particular
formula, the acetic acid alone (see part 5) showed a very different behaviour
than the diluted Acetic Lugol’s.
But... the best fixatives formulae are
not of course simple, solitary reagents (they are a formula because of this, aren't they?). They are a mixture of reagents, each of
which brings its special mode of action. They are synergistic agents.
And possibly is an effect of this type
which is responsible for the last good images of some onion skin cells, fixed
and stained with the 0.1% Acetified Lugol’s solution. (Acetic-Lugol/10)
We have seen the same phenomenon using
Ethylic and Acetic in the Clarke’s fixative. Each component completes the
action of the other.
Well! Acetic-Lugol/10 is excellent. There are other safe fixatives which merit to be tried on the “onion skin”, but this one has proved to work.
At the end, it's probable that you
think, correctly, that I can live with a few air bubbles spoiling my images taken
with the 4x objective! I give up!
But don’t miss the next article. As Scheherazade used to say: “there is another tale”