|
|
A Gallery of Vanillin Photomicrographs (using
polarized light illumination) |
|
|
A Gallery of Vanillin Photomicrographs (using
polarized light illumination) |
Vanillin
(4-hydroxy-3-methoxybenzaldehyde), is one of the most easily
identifiable flavourings throughout the world. Its pleasant taste
and smell make it a useful ingredient in the formulation of food
flavourings, fragrances, pharmaceuticals and perfumes. In fact my
most recent toothpaste purchase is advertised as having a “refreshing
vanilla mint” flavour!
The compound was first obtained
from the seed pods of the orchid “Vanilla planifolia”.
The extract from the pods is 98% vanillin and 2% other complex
molecules. The 2% actually causes the extract to have a
noticeably different taste than pure vanillin. This is the reason
that gourmet chefs extol the virtues of “good” vanilla over the
“inferior” synthesized variety. Today only 1% of vanilla is
obtained from orchid pods. Much of the rest is obtained as a
byproduct of the pulp and paper industry. Chemical synthesis
accounts for the remainder.
Since the melting temperature of
pure vanillin is low, about 82 oC, a melt specimen for
observation with the polarizing microscope, is easy to prepare. A
small quantity of the compound, a white to pale yellow crystalline
solid, is placed on a microscope slide, covered by a cover-glass, and
heated gently with an alcohol lamp. When the crystals melt
completely and form a thin layer between slide and cover-glass, the
slide is removed from the vicinity of the flame and allowed to cool.
I must say that the preparation of
vanillin melt specimens is a particularly pleasant experience.
Many of the chemicals that I work with while preparing articles for
Micscape have extremely unpleasant odors. One of the most
malodorous is elemental sulphur.
It should be noted however that
caution should be exercised while using pure vanillin. The MSDS safety
document for the compound states the following.
WARNING!
MAY BE HARMFUL IF SWALLOWED, INHALED OR ABSORBED THROUGH SKIN. MAY
CAUSE IRRITATION TO SKIN, EYES, AND RESPIRATORY TRACT.
CARBON DIOXIDE AND CARBON MONOXIDE MAY FORM WHEN
HEATED TO DECOMPOSITION. (Carbon monoxide is an
extremely poisonous gas.)
The structural formula and
molecular shape of vanillin are shown below. (Both illustrations
were prepared using HyperChem Pro
software.
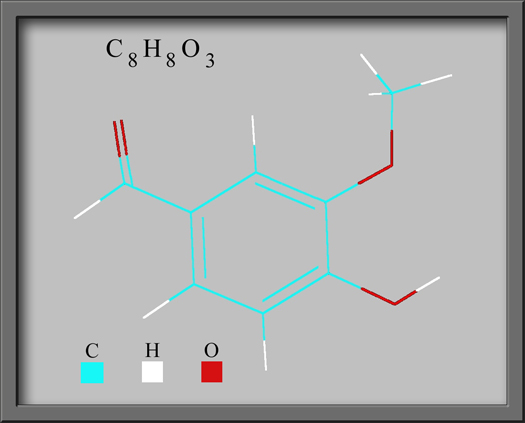
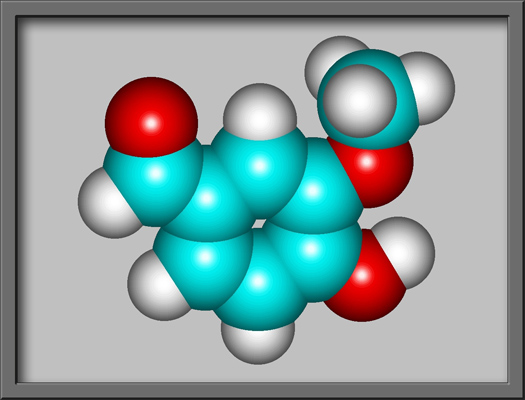
As the molten compound
re-crystallizes, some sections may have greater thickness than
others. This difference is one of the factors that cause
different colour pallets to be displayed between crossed polars.
The light gray sections in the image below are particularly thin
crystal formations.

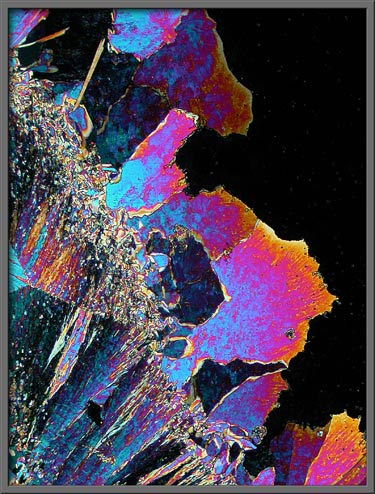
The three images that follow were
obtained while the melt was re-crystallizing. Great speed is
required since the crystal growth front seen in the image is advancing
at an alarming speed. Seconds later, the entire field would be
covered with crystals. (Crossed
polars)


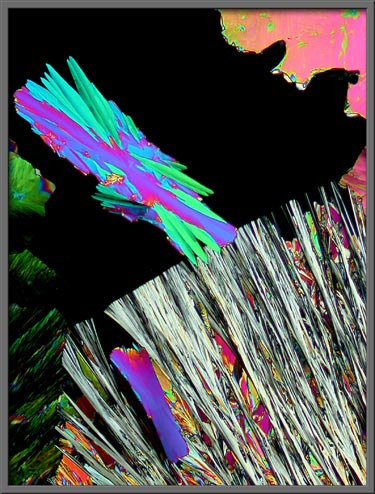
Occasionally circular growth
fronts, called spheroliths are
observed. (Crossed polars)




The higher magnification image
below shows a rectangular growth that has started to encroach into the
edge of a large spherolith. (Crossed
polars)
Interesting patterns are often seen
at the center of spheroliths. (Crossed
polars)


Compensators called “wave-plates”
can be inserted into the light path in a polarizing microscope in order
to change the colouration of the image. The two images that
follow are of exactly the same field. (Crossed
polars + two lambda/4 plates – One lambda/4 plate was
rotated to produce the colour difference.)


Here is a second example of the use
of compensators. Again the field shown is the same for both
images. (Left image: Crossed polars + two lambda/4 plates
– Right image:
Crossed polars + lambda/4 plate +
lambda plate)

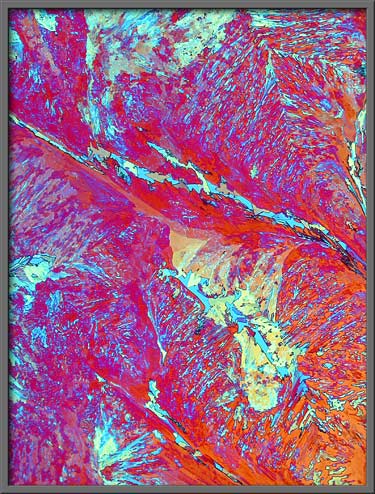
The three images that follow show
typical vanillin fields. (Note:
The first image in the article is identical to the first image below,
however, it was post-processed using Adobe
Photoshop’s “Invert
(colour)” command. A “Levels”
adjustment was also performed in order to increase the contrast.)



Finally, here are two fields
showing serpentine gaps that often occur in melt specimens. (Left image: Crossed polars + lambda/4 plate + lambda
plate – Right
image: Crossed polars + two
lambda/4 plates)


The
Mexican Aztecs first used the vanilla plant as a flavouring in the 14th
century. It’s not surprising that its popularity remains
undiminished in the 21st century!
Photomicrographic Equipment
The images in the article were
photographed using a Nikon Coolpix 4500 camera attached to a Leitz
SM-Pol polarizing microscope. Images were produced using a
polarizing condenser. Crossed polars were used in all
images. Compensators, ( lambda and lambda/4 plates ), were
utilized to alter the appearance in some cases. A 2.5x, 6.3x, 16x
or 25x flat-field objective formed the original image and a 10x
Periplan eyepiece projected the image to the camera lens.
Published in the
November 2007 edition of Micscape.
Please report any Web problems or
offer general comments to the Micscape
Editor.
Micscape is the on-line monthly magazine
of the Microscopy UK web
site at Microscopy-UK
© Onview.net Ltd, Microscopy-UK, and all contributors 1995 onwards. All rights reserved. Main site is at www.microscopy-uk.org.uk with full mirror at www.microscopy-uk.net .