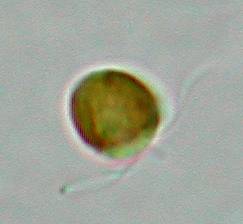
 Not
surprisingly, the tiny algal flagellate Phaeocystis shows up
massively. As a swarmer, it has two flagella and a short little hair
in between. This hair is quite different in structure from the
flagella, and is called a haptonema. It is thought to be involved in
food gathering, and all algae that have this structure belong to the
class of haptophytes. Nevertheless, it is quite rudimentary in
Phaeocystis and I doubt whether it is functioning as such in
this species.
Phaeocystis
sheds its flagella easily and then starts secreting a jelly like
substance. Massive colonies of cells in mucilage are floating through
the water, often washing ashore in huge foaming waves, hence the
Dutch name of Schuimalg (Foam algae) for this nuisance organism.
Not
surprisingly, the tiny algal flagellate Phaeocystis shows up
massively. As a swarmer, it has two flagella and a short little hair
in between. This hair is quite different in structure from the
flagella, and is called a haptonema. It is thought to be involved in
food gathering, and all algae that have this structure belong to the
class of haptophytes. Nevertheless, it is quite rudimentary in
Phaeocystis and I doubt whether it is functioning as such in
this species.
Phaeocystis
sheds its flagella easily and then starts secreting a jelly like
substance. Massive colonies of cells in mucilage are floating through
the water, often washing ashore in huge foaming waves, hence the
Dutch name of Schuimalg (Foam algae) for this nuisance organism.
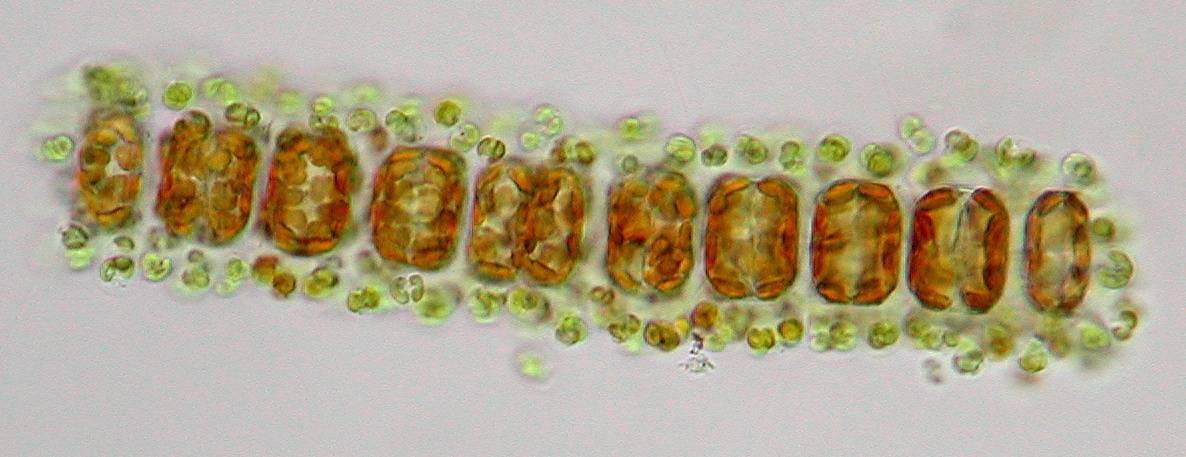
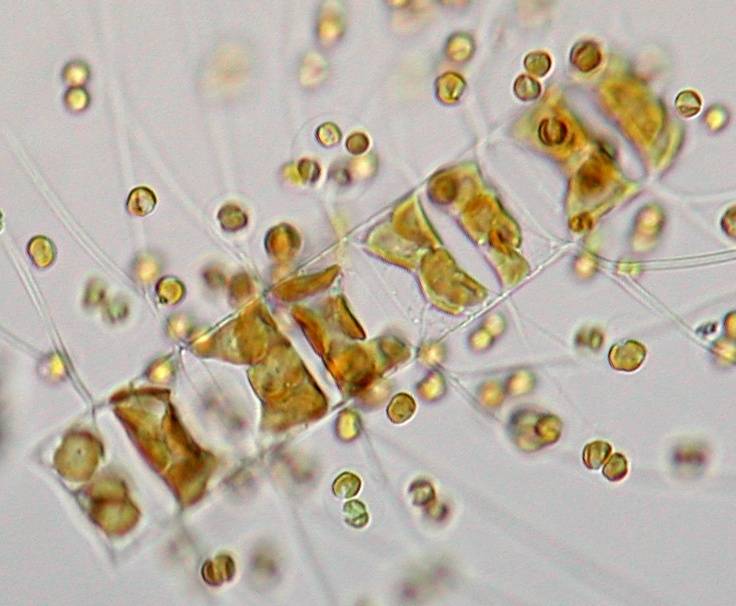
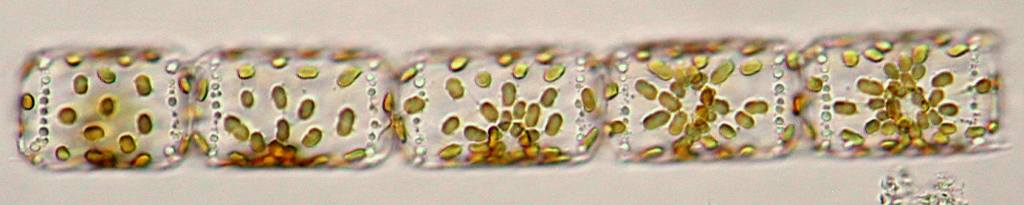
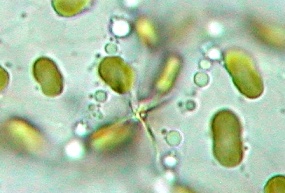 Also
typical for this species are the two connection points between the
cells that link them together. This is actually a good determination
characteristic that is best seen at the end of the chains.
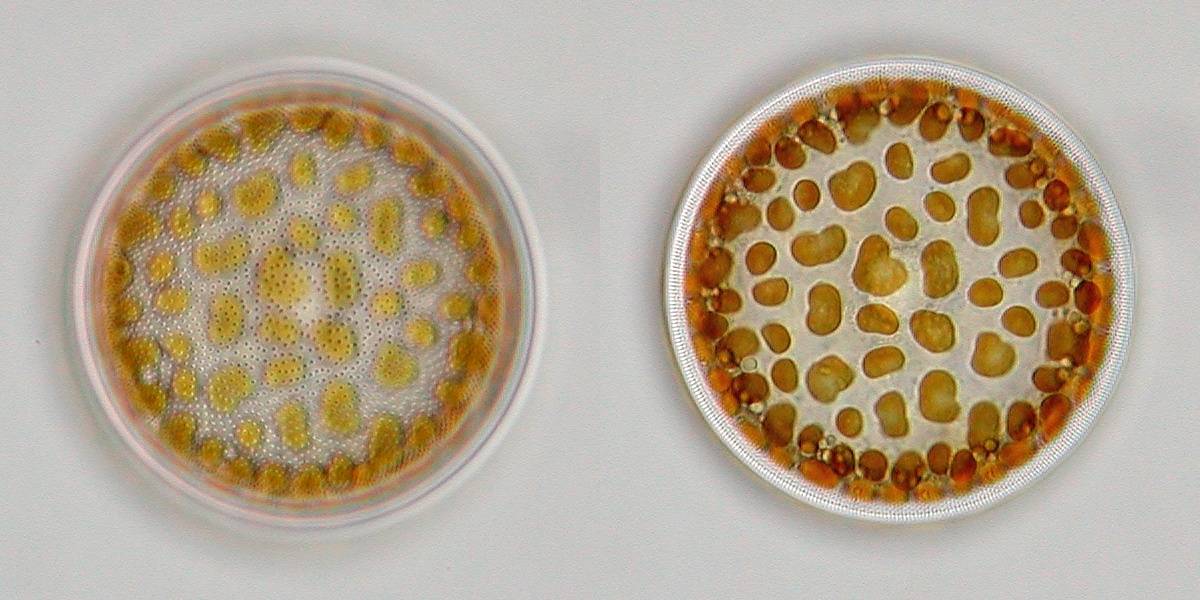
One
of the nicest occurring centric diatoms in our coastal waters is
Actinocyclus octonarius, a very beautiful solitary species
which shows a dotted line pattern around the edge when focussing on
the middle of the diatom. Also the pore (areolae) pattern on the
valvar surface is characteristic for this species.
Also
typical for this species are the two connection points between the
cells that link them together. This is actually a good determination
characteristic that is best seen at the end of the chains.
One
of the nicest occurring centric diatoms in our coastal waters is
Actinocyclus octonarius, a very beautiful solitary species
which shows a dotted line pattern around the edge when focussing on
the middle of the diatom. Also the pore (areolae) pattern on the
valvar surface is characteristic for this species.
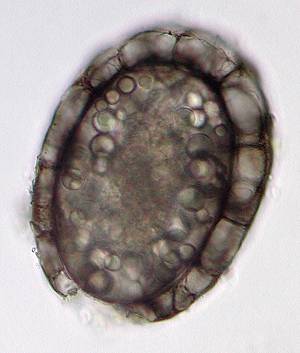 Not
surprisingly, with plentiful food around, predators are on the hunt
as well! One of the largest predatory dinoflagellates that I found
was Polykrikos schwartzii. In fact, I first came across the
cyst of this species, a thick walled and darkly brown coloured
resting spore.
Dinoflagellates
are a bit of a strange group, as the name sounds, from a long past.
Some of them are heavily armored with cellulose plates, some appear
naked. Generally, one flagel is running through a deep groove along
the body, the second one is trailing behind. In fact Polykrikos
has several of those grooves around its flexible body, indicating a
multicellular origin, a pseudo-colony in which several nuclei share
the same protoplasma. These large nuclei are visible in the next
picture, where the genetic material shows as two threaded masses of
permanently condensed chromosomes.
Not
surprisingly, with plentiful food around, predators are on the hunt
as well! One of the largest predatory dinoflagellates that I found
was Polykrikos schwartzii. In fact, I first came across the
cyst of this species, a thick walled and darkly brown coloured
resting spore.
Dinoflagellates
are a bit of a strange group, as the name sounds, from a long past.
Some of them are heavily armored with cellulose plates, some appear
naked. Generally, one flagel is running through a deep groove along
the body, the second one is trailing behind. In fact Polykrikos
has several of those grooves around its flexible body, indicating a
multicellular origin, a pseudo-colony in which several nuclei share
the same protoplasma. These large nuclei are visible in the next
picture, where the genetic material shows as two threaded masses of
permanently condensed chromosomes.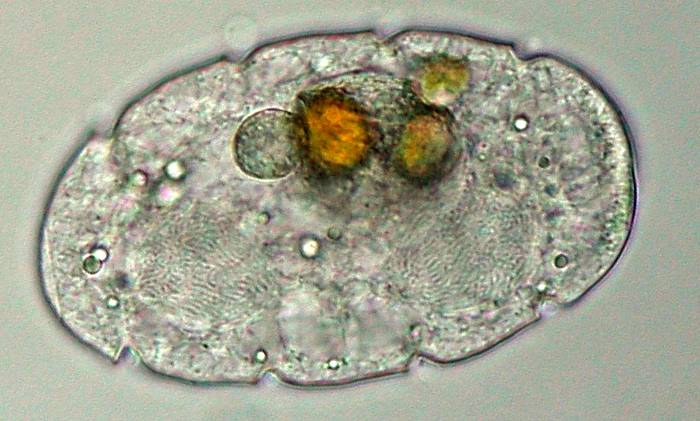
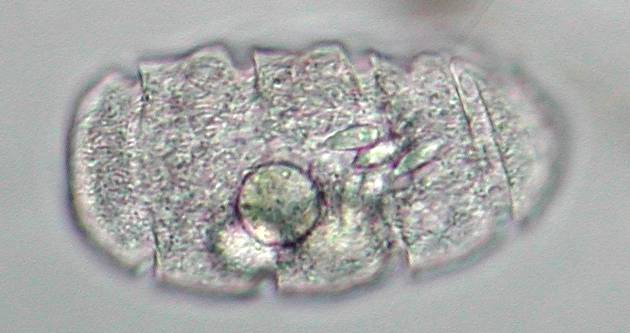
 Of
course a cell doesn’t want to loose these nematocysts too
easily, so conditions have to be exactly right before these kind of
specialized organelles are triggered and fired off. But what these
conditions are, and how this organism senses its prey, is still a bit
of a mystery. In fact, the organism itself is quite fragile, and
after some examination in a crowded micro-aquarium under the
microscope it quickly started to round off, and finally burst. Some
of those nematocysts landed on the floor of the micro-aquarium, so I
got a good look at the remarkable structure of this single organelle,
which is only 13 µm long.
Of
course a cell doesn’t want to loose these nematocysts too
easily, so conditions have to be exactly right before these kind of
specialized organelles are triggered and fired off. But what these
conditions are, and how this organism senses its prey, is still a bit
of a mystery. In fact, the organism itself is quite fragile, and
after some examination in a crowded micro-aquarium under the
microscope it quickly started to round off, and finally burst. Some
of those nematocysts landed on the floor of the micro-aquarium, so I
got a good look at the remarkable structure of this single organelle,
which is only 13 µm long.
Technicalities
Author is working at an ecological research and consultancy company (www.koemanenbijkerk.nl) in the north of The Netherlands. Preserved seawater samples for the Dutch phytoplankton monitoring program are taken by the Directorate-General for Public Works and Water Management, and analysed in our laboratory. Also live samples are taken on a regular basis, which gives an indication of the biodiversity, and we’re closely watching for any toxic or nuisance algae that show up. As live samples can be in transport for several days before analysis, this can only considered to be semi-quantitative, and an example of the report for this sample can be found here. Analysis is carried out on inverted microscopes at 200x with the sample in so-called sedimentation cuvettes with a volume of around 1 ml. Images were taken with 20/0.7 and 60/1.4 lenses in brightfield. Thanks to Bert Wetsteyn (DG for Public Works and Water Management) for friendly permission of the use of analysis results and helpful comments.All comments to the author René van Wezel are welcomed.