Notes
on using a Minolta Scan Elite 5400 for scanning microscope slides.
Comparisons
with the Nikon Coolscan design.
by David Walker, UK

|
Notes
on using a Minolta Scan Elite 5400 for scanning microscope slides. by David Walker, UK |
|
In an August 2004 article and later image gallery I shared my experiences as a hobbyist on using a secondhand Nikon Coolscan IV for scanning microscope slide specimens. This is a 2900 dpi 35mm film scanner and its design works well for microscope slide scanning with a homemade holder, thus avoiding the need for Nikon's own expensive microscope slide attachment.
I recently had a chance to borrow a colleague's Minolta Scan Elite 5400 dpi 35mm slide scanner and was interested to see how it performed as a microscope slide scannerŚwith nearly twice the resolution as the Coolscan IV it should capture detail much better and has a completely different design to the Coolscan. I couldn't find any information on the Web either by Minolta or others regarding the 5400's potential use as a microscope slide scanner, so hopefully these trials and comparisons may be of interest.
The microscope slide handling comparisons should also apply to Nikon's current model, the Coolscan V as it appears to retain the same 35 mm film handling attachments as the IV. For the hobbyist, a slide scanner's potential for microscope slide scanning is a bonus to its normal film use, although apparently in professional circles, 35 mm film scanners are widely used for microscope slide scanning (see refs. to papers kindly sent by readers in the earlier article).
Physical handling of microscope slides compared
Nikon Coolscan (see August 2004 article for details)


Scanning method: In
the manual, Nikon state that the scanner (see title image) can be vertical
or horizontal for film scanning. However, it must be vertical for
microscope slides if a homemade holder is used, to ensure the slide is horizontal. The slide and
holder
remains stationary while scanning. Coverslip facing down (shown
face up in above images).
Microscope slide holder used: Nikon's supplied 35 mm
slide holder (right above) and a modified 35 mm plastic slide mount (left
above) allows safe
scanning of microscope slides. Care is required when inserting and
removing microscope slides as gravity is partly being used to ensure alignment.
Minolta 5400
In the manual, Minolta advise against using the scanner horizontally when film scanning, so a microscope slide if scanned will be vertical. Visual inspection of the Minolta confirmed that the scanner's optics and mechanics stayed well away from the film plane during film scanning. Microscope slide scanning should therefore be safe as long as a glass slide was mounted in the film plane and securely held so that there was never a possibility of it falling out into the scanner. This is particularly important for the Minolta as, unlike the Coolscan, the 35 mm slide film holder moves during scanning.
|
After some careful trials the following method seems to the author to be one safe and reliable way of holding microscope slides (but see disclaimer below!). The same modified 35 mm plastic slide film
holder was used as for the Coolscan. This was used with
the Minolta 35 mm slide film holder shown below right (this is supplied
with the scanner). These strips have to be chosen with care; they need to be just thick enough to hold the slide firm when holder is closed but without putting stress on the glass slide or prevent the slide holder from securely closing. (The author used two strips of 1 mm thick black foam with backing strip intact used for repairing the light tight seals on cameras.) |
|
| Using the above method, when the 35 mm film holder is closed the microscope slide is very secure, shaking the holder does not move it. So in this respect in the author's opinion, this is a more secure scanning setup for microscope slides than for the Coolscan with homemade holder. Although not tried, the recess in the 35 mm film holder allows crossed polar imaging to be carried out by temporarily mounting filters either side of the slide, as carried out successfully for the Coolscan IV (see earlier article). |
|
Software: both the Nikon Coolscan and Minolta 5400 have excellent and versatile software. I had a preference for the Minolta software, it seems better integrated than the Nikon's. Click image left below for a view of Minolta's scanning interface. See earlier article for a screen shot of Nikon's.
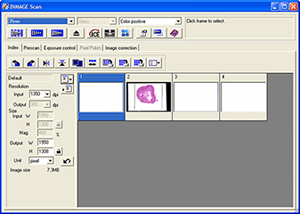
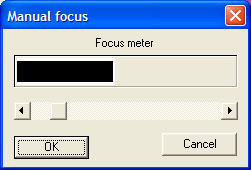
Minolta
scanning software interface (Dimage Scan 1.1) and detail of manual focus bar.
Focussing: The Nikon Coolscan's autofocus seems to cope well with most microscope slide subjects. There's also an option to force it to autofocus on a selected area of a slide. The Coolscan's manual focus is awkward to use with no realtime indication of how adjustments affect focus.
The Minolta seemed to work better for microscope slides in manual focus mode which was straightforward as a selected area to focus on is picked on the slide (e.g. a high contrast area) and a real time 'focus meter' shown above is seen, the sliding bar is adjusted until the black bar reaches a maximum in the white box.
Image
comparisons
Note:
This is more a comparison of increasing the dpi rather than Minolta versus Coolscan
as the comparable model to the Minolta is the Coolscan V (4000 dpi with Nikon
Scan 4 software) not the Coolscan IV
(2900 dpi, Nikon Scan 3.0). The links to masters are unretouched jpegs
at Photoshop Elements compression setting 10 to keep file size down.
Crops
of master scans shown appear somewhat soft as they are small areas of large
images that wouldn't normally be inspected this close on a screen.
Conclusion: From a short trial the Minolta Dimage Scan Elite 5400 works well for safely scanning microscope slides with a homemade microscope slide holder. For subjects with fine detail such as plant sections and histology the Minolta 5400 reveals more detail than the Coolscan IV 2900 dpi and also gives the opportunity to scan selected smaller areas.
However, a film scanner offers most potential for imaging microscope slides when the subject nearly fills the 35 mm frame area, as imaging subjects this large would require multiple stitching with microscopy. When the subject only partly fills the frame area, the usable pixels even for a 5900 dpi model plummets and microscopy imaging could cover the same area with some limited stitching at a higher quality.
As commented in the earlier article, a modest low power microscope objective outperforms a scanner's resolution capabilities, even for a 5400 dpi model. A LOMO 3.5x NA0.10 planachromatic objective has a theoretical resolution of ca. 3 microns cf the Minolta's 4.7 Ám theoretical and ca. 7-10 Ám in practice. If very low power microscopy imaging was of interest, a 1x NA0.04 planachro compound microscope objective often sells quite cheaply on eBay and have a theoretical resolution of ca. 7 Ám, i.e. comparable imaging to the expensive film scanner at a fraction of the cost. From the author's trials, a 1x objective typically has a horizontal field of view at a 35 mm SLR film plane of 12 x 8 mm (7x eyepiece) so ca. four to six image stitches would give a comparable field of view to the scanner's max. of 36 x 24 mm. The current crop of high megapixel consumer digicams with 'super macro' facilities should also give good imaging.
In professional applications, where convenience and speed of imaging large subjects are important, the benefits of the film scanner are probably more pertinent.
Comments to the author David Walker are welcomed.
Disclaimer: The above information is offered in good faith but neither the author nor website owner accepts any responsibility for damage to a slide scanner for adopting these techniques. It's 'user beware' and any damage would invalidate the maker's guarantee.
Published in the May 2005 edition of Micscape.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK web site at Microscopy-UK
ę Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is
at www.microscopy-uk.org.uk
with full mirror
at www.microscopy-uk.net
.