STAINING
AND OTHER METHODS FOR ENHANCING THE OBSERVATION OF CELLULOSE
LACQUER ROCK PEELS.
BY
KEITH W. ABINERI, UK.
42
West Borough, Wimborne, Dorset BH21 1NQ, UK
Tel. 01202 885547
Introduction.
With the previously
discussed unstained cellulose lacquer rock peels, a small
area of the rock surface (ca. 2 cm2) was prepared
initially by grinding, flatting and polishing with abrasive
papers Nos. 320, 600 and 1200 respectively. Between each stage
the rock surface was rinsed thoroughly with distilled water. Then
generally 2% hydrochloric acid was used to etch the rock surface
i.e. two or three drops of the acid were spread over the area and
allowed to react for about 12 - 15 seconds, followed by rinsing
with distilled water. Finally, before applying the cellulose
lacquer, the rock surface was treated with a few drops of acetone
(propanone) to remove the last traces of water. These carefully
prepared unstained peels could then be examined using
brightfield, darkfield, phase contrast, parallel polarizing
plates (PPL) or crossed polarizing plates (XPL) types of
illumination. With my Nikon substage condenser it was possible
also to use darkfield illumination combined with XPL, to enhance
the observation and photography of the walls of the chambers in
minute Upper Chalk Foraminifera skeletons, or tests.
However during late 1986 and early l987 the first experiments
were completed by the writer to combine the cellulose lacquer
rock peel technique with a well established method for staining
carbonate rock material. This latter method was published in 1984
by A.E.Adams, W. S. MacKenzie and C. Guilford1,
in their excellent "Atlas of sedimentary rocks under the
microscope" in the References and refers to the staining of
cellulose acetate peels. The very high resolution obtained with
the cellulose lacquer unstained peels, encouraged me to use this
method of staining with some modifications.
Modifications to produce stained cellulose lacquer rock
peels.
In place of the simple acid etching solution the following is
used :-
Solution (A):
10 cm3 0.5 Molar Hydrochloric Acid.
20 cm3 Distilled Water.
36 mg Alizarin Red S.
This solution may be held in stock as it is quite stable. Just
before staining the rock surface, 4.0 x 10-2 grams of
potassium ferricyanide, K3Fe(CN)6 is added
to 5 cm3 of solution (A) and dissolved. This mixture
will stain many rock samples since only a few drops are needed
for about 2 cm2 of each rock surface, but it should be
used as soon as possible.
The quantities of Alizarin Red S and potassium ferricyanide can
be estimated in the absence of an accurate chemical balance. For
this purpose the following approximate "pack densities"
might be useful:-
Alizarin Red S 0.5 grams/cm3.
Potassium Ferricyanide 1.5 grams/cm3.
The 0.5 Molar hydrochloric solution can be obtained from
chemical suppliers such as B.D.H., who stock also Alizarin Red S
and potassium ferricyanide. All these chemicals must be handled
with care to avoid contact with skin or eyes.
After the normal grinding, flatting, polishing and thorough
rinsing with distilled water, the 2 cm2 area of the
dried rock surface is treated with a few drops of the combined
etching and staining solution using a small pipette, or dropper.
With carbonate rocks or calcareous microfossils or nannofossils
in clays or shales, the staining period can be from 20 seconds
and 60 seconds, depending on the nature of the rock and practical
experience. During this period the solution on the rock surface
should be very gently agitated, using the dropper pipette. Now
the stained surface must be very thoroughly rinsed with distilled
water to remove all traces of the reagents. No detergent must
be used.
After drying, acetone2 is applied to remove the last
traces of water. Finally the cellulose lacquer is applied and
used to prepare the peel and finished microscope slide in the
usual manner. During these latter stages avoid touching the
stained rock surface.
The following staining colours are obtained.
Pure Calcite3 : Pink to
Red-Brown.
Ferroan Calcite4 : Mauve to Deep Blue depending on
the Iron (II) content.
The intensity of these colours depends also on grain size.
With smaller grain size the intensity is greater. Also :-
Pure Dolomite5 : No stain.
Ferroan Dolomite6 : Very Pale Blue to Green.
Silica7 and Silicates8 : No stain.
The chemistry of this staining procedure will be discussed in
a later article.
The six accompanied illustrations and notes cover the following
aspects :-
Figures (1) to (4) inclusive : Examples of stained cellulose
lacquer rock peels.
Figure (5) : An example of a stained cellulose lacquer peel as
seen with darkfield illumination.
Figure 6 : An example of an unstained cellulose lacquer peel as
seen with crossed polarizing plates (XPL).
Figures (1) to (4) show individual differential staining of
nannofossils and aid in their detection.
Figure (5) shows the effect of oblique illumination on the
appearance of a stained cellulose lacquer peel.
Figure (6) shows numerous minute optical figures of coccoliths
and other calcareous nannofossils9 embedded in
transparent kerogen10 organic residues, which have
been removed from the rock surface by the peeling process.
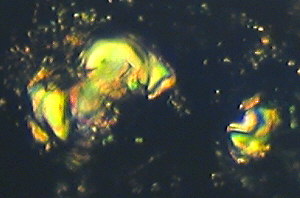
Examples of stained cellulose lacquer rock peels i.e. Figures
(1) to (4).
| Figure (1).
Differentially stained marine nannofossils (coccoliths
and coccolithophores) in a layer of coccolithic
limestone, from the Upper Jurassic, Upper Kimmeridge
Clay, Freshwater Steps Stone Band. Map Reference
SY.943.772. Freshwater Steps, west of Hounstout Cliff,
Dorset coast. Objective N.A. = 0.65. Total field area of
the image ca. 210 microns X 150 microns. |
 |
Figure (1) is an example of both replica and removed thin layer
type of images. It shows an area of "golden" coloured
coccoliths which are really not stained, because they are buried
in transparent kerogen, which gives them this appearance. The
pink areas and nannofossils are due to pure calcite composition.
The dark blue areas and nannofossils indicate ferroan calcite
composition. The black objects suggest fusain11
(carbon) or pyrites grains12. Figure (1) implies the
occurrence of an algal "bloom" of coccolithophore
marine phytoplankton in remote Kimmeridgian times, with
associated mixed aerobic13 and anaerobic14
conditions on a minute scale. The picture was obtained with
brightfield illumination and PPL.
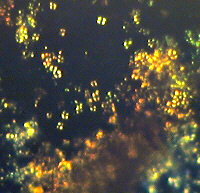
| Figure (2).
This picture was derived from the same stained cellulose
lacquer rock peel as used with Figure (1), but covering a
different area with increased magnification. Here
oil-immersion objective N.A. = 1.25 was used. The total
field area of the image = ca. 90 microns X 70 microns. |
 |
Here again Figure (2) is an example of both replica and removed
thin layer type of images. It was obtained with brightfield
illumination.
The conclusions from examination of Figure (2) are similar to
those from Figure (1). It should be noted that the staining of
these peels does produce some damage to the nannofossils by the
action of the acid (dilute hydrochloric) on the calcareous
material. This results in the loss of some clarity with some
highly magnified stained images, when compared with similar
unstained peel images. However the complex staining on such a
minute scale on Figure (2), does appear to represent an equally
complex chemistry (or biochemistry) of these largely biogenic
sediments.
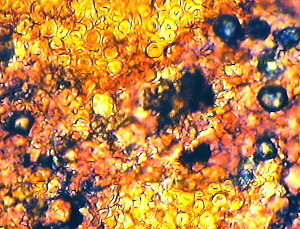
| Figure (3).
Here we have larger nannofossils on a complex background
of numerous much smaller nannofossils, many of which are
embedded in transparent kerogen, and are unstained
"golden" coccoliths. This picture is from the
Upper Jurassic, Upper Kimmeridge Clay, Freshwater Steps
Stone Band. Map Reference : SY. 943.772. Freshwater
Steps, west of Hounstout Cliff, Dorset coast. Objective
N.A. = 0.65. Total field area of the image = ca. 270
microns X 200 microns. |
 |
Figure (3) was obtained with brightfield illumination and PPL.
It shows land forest swamp debris mixed with marine nannofossils
and microfossils, typical of Kimmeridgian times.
 It is a further
example of both replica and removed thin layer type of images.
The staining on this peel is uneven as is shown on this small
area, probably due to kerogen and other organic residues present
in the sediment. The prominently red stained calcisphere15
(shown right) is interesting with its outer pale green layer.
This suggests a pure calcite interior with an outer layer of
ferroan dolomite. The slow change of calcite (diagenesis) to
dolomite has occurred in a number of stone bands in the Dorset
Kimmeridge Clay. This may have biological origins. The further
conversion to ferroan dolomite suggests some anaerobic conditions
in this particular case, since minute quantities of the reduced
form of iron (II) appear to have displaced calcium in the
carbonate rock, in the nannofossil. Elsewhere on this peel
calcispheres have been found showing a deep blue interior,
indicative of ferroan calcite. These are further evidence for
anaerobic conditions on a minute scale. The presence of fusain
and pyrites buried in the kerogen on Figure (3) suggests another
reason for the anaerobic environment.
It is a further
example of both replica and removed thin layer type of images.
The staining on this peel is uneven as is shown on this small
area, probably due to kerogen and other organic residues present
in the sediment. The prominently red stained calcisphere15
(shown right) is interesting with its outer pale green layer.
This suggests a pure calcite interior with an outer layer of
ferroan dolomite. The slow change of calcite (diagenesis) to
dolomite has occurred in a number of stone bands in the Dorset
Kimmeridge Clay. This may have biological origins. The further
conversion to ferroan dolomite suggests some anaerobic conditions
in this particular case, since minute quantities of the reduced
form of iron (II) appear to have displaced calcium in the
carbonate rock, in the nannofossil. Elsewhere on this peel
calcispheres have been found showing a deep blue interior,
indicative of ferroan calcite. These are further evidence for
anaerobic conditions on a minute scale. The presence of fusain
and pyrites buried in the kerogen on Figure (3) suggests another
reason for the anaerobic environment.
A further largely buried object lies in the central area of
Figure (3). This is covered with coccoliths and other
nannofossils and has a diameter of circa. 58 microns. It is
believed to be a marine microfossil, a Dinoflagellate Cyst16.
Many species of these are found in the various Kimmeridge Clay
beds, often buried in the peeled kerogen layers. They are best
studied by complete separation from the rock by palynology
techniques using concentrated hydrofluoric acid to remove all the
mineral contents and preparing a microscope slide of the organic
residues only.
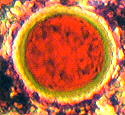
| Figure (4).
Here we have a minute part of a stained cellulose lacquer
peel from the Cretaceous, Upper Chalk, Actinocamax
Quadratus Zone. Map Reference SY.851.802. West of Arish
Mell on the cliff, Dorset coast. Oil-immersion objective
N.A. = 1.25. Total field area of the image 80 microns X
60 microns. |
 |
It shows the probable cell-division of a Chalk Calcisphere on a
background of numerous Chalk Coccoliths, some of which are
damaged or partly buried. The differential staining is
interesting and appears to be due to differences in texture or
grain size. Furthermore the complete lack of any blue staining
implies the absence of any Ferroan Calcite and therefore the
predominatingly aerobic environment. We may deduce from this that
the Upper Chalk was deposited in shallow open seas in the
presence of abundant oxygen, which stimulated the photosynthesis
for the growth of coccolithophores in the marine plankton.
On Figure (4) the diameters of the two dividing calcispheres are
about 11 - 14 microns. This picture was obtained with brightfield
illumination and one polarizing plate above the objective.
Darkfield illumination used with a stained cellulose lacquer
peel.
| Figure (5).
Here we have a small part of a stained cellulose lacquer
peel from the Lower Jurassic, Lower Lias, Belemnite
Marls, Charmouthian. Map Reference : SY.380.927. East of
Charmouth, Dorset coast. Objective N.A. = 0.25. Total
field area of the image = circa. 1100 microns X 820
microns. |
 |
It is another case of both replica and removed layer type of
images. The dark-field form of illumination blocks the light from
direct transmission though the peel and enhances oblique
lighting. This accentuates the blue staining.
Objects are seen by virtue of scattered light. The largest
object on Figure (5) is the section (transverse) of a small
Crinoid stem17. This has a diameter of circa. 840
microns. Darkfield illumination enhances its internal structure.
The blue colour is thought to be due to the inclusion of Ferroan
Calcite, indicating an anaerobic environment. The black and brown
mottled area is believed to be due to microcrystals of Pyrites,
another indication of anaerobic conditions. Crinoids are a small
species of the Echinodermata and are found also in remote times
in limestones of the Carboniferous period.
Darkfield illumination used with stained cellulose lacquer peels
shows many interesting aspects. It often tends to increase the
contrast in the images and can help to distinguish between dark
wood fusain and pyrities, which so often occur together.
Crossed Polarizing Plates (XPL) used with an unstained
cellulose lacquer peel.
| Figure (6).
Here we have two minute parts of an unstained lacquer
peel, observed under XPL, to show optical figures of
calcareous nannofossils embedded in transparent kerogen
and other organic residues. The peel is from the Upper
Jurassic, Upper Kimmeridge Clay, Freshwater Steps Stone
Band. Map Reference : SY. 943.772. Freshwater Steps, west
of Hounstout Cliff, Dorset coast. Objective N.A. = 0.65.
The total field area of the images = ca. 120 microns X 80
microns and ca. 130 microns X 120 microns. |
It shows removed material only in the form of real calcareous
nannofossils which produce optical figures under XPL. Under this
type of illumination no replicas are seen. The white optical
figures indicate uncovered calcareous nannofossils, whereas the
pale yellow optical figures are embedded in transparent kerogen,
(cf. the "golden" coccoliths shown on Figures (l), (2)
and (3)). Clearly most of the optical figures on Figure (6) are
derived from the coccolith calcite plates which have come from
the breakdown of the numerous coccolithophore unicellular plants
(marine phytoplankton). These microscopic optical figures are an
important characteristic of the crystalline structure of the
calcite in the coccoliths. This technique can be used to study a
large variety of coccolithic limestones, throughout geological
time.
Notes and References.
1. "Atlas of sedimentary rocks under the microscope".
This excellent full-colour handbook by A.E. Adams, W.S. MacKenzie
and C. Guilford 1984, published by Longman, has an appendix on
the staining of acetate-peels.
2. Acetone (propanone) : a very volatile solvent
completely miscible with water.
3. Pure Calcite : crystalline calcium carbonate.
4. Ferroan Calcite : crystalline calcium carbonate with a very
small proportion of the calcium displaced by iron(II) in the
crystal lattice. This occurs under anaerobic environments.
5. Pure Dolomite : a compounded carbonate of calcium and
magnesium.
6. Ferroan Dolomite : Dolomite with a very small proportion of
calcium displaced by iron(II).
7. Silica : Silicon dioxide in various forms e.g. quartz grains,
sand grains etc.
8. Silicates : frequent ingredients of numerous rock minerals.
9. Calcareous nannofossils : Nannofossils largely composed of
calcium carbonate. Many forms belong to the Coccolithophyceae.
10. Kerogen : A solid complex organic material which yields
petroleum type hydrocarbons under heat and pressure.
11. Fusain : carbonaceous material derived from decaying
vegetation or wood.
12. Pyrites : a most widespread sulphide of iron. A sure sign of
anaerobic sediments.
13. Aerobic : in the presence of oxygen.
14. Anaerobic : in the absence of oxygen.
15. Calcispheres : Minute hollow microfossils and nannofossils of
calcareous composition. Found frequently in chalk and limestone
sediments. They have existed in differing forms since the
Devonian Period (circa. 380 million years ago).
16. Dinoflagellate Cysts : a common group of microfossils, which
have existed since early Jurassic times (circa. 213 million years
ago).
17. Crinoids : These are microscopic animals of the Echinodermata
group. They are species Crinoidea and their fossils occur
frequently in marine limestones.
Editor's note: Some of the quality of the
author's original 35mm slides is lost in the scanned and
compressed web images. Comments to the author are welcomed, who
can be contacted at the above address or comments can be passed
on via the Micscape Editor, see contact on magazine index.
Article prepared for the Web by David
Walker.
© Microscopy UK or their
contributors.
Published in the March 1999 edition of Micscape
on-line and archived at
http://www.microscopy-uk.net/mag/artmar99/kamast2.html
Please report any Web problems
or offer general comments to the Micscape Editor,
via the contact on current Micscape Index.
Micscape is the on-line monthly
magazine of the Microscopy UK web
site at Microscopy-UK
WIDTH=1
© Onview.net Ltd, Microscopy-UK, and all contributors 1995 onwards. All rights
reserved. Main site is at www.microscopy-uk.org.uk with full mirror at www.microscopy-uk.net.



 It is a further
example of both replica and removed thin layer type of images.
The staining on this peel is uneven as is shown on this small
area, probably due to kerogen and other organic residues present
in the sediment. The prominently red stained calcisphere15
(shown right) is interesting with its outer pale green layer.
This suggests a pure calcite interior with an outer layer of
ferroan dolomite. The slow change of calcite (diagenesis) to
dolomite has occurred in a number of stone bands in the Dorset
Kimmeridge Clay. This may have biological origins. The further
conversion to ferroan dolomite suggests some anaerobic conditions
in this particular case, since minute quantities of the reduced
form of iron (II) appear to have displaced calcium in the
carbonate rock, in the nannofossil. Elsewhere on this peel
calcispheres have been found showing a deep blue interior,
indicative of ferroan calcite. These are further evidence for
anaerobic conditions on a minute scale. The presence of fusain
and pyrites buried in the kerogen on Figure (3) suggests another
reason for the anaerobic environment.
It is a further
example of both replica and removed thin layer type of images.
The staining on this peel is uneven as is shown on this small
area, probably due to kerogen and other organic residues present
in the sediment. The prominently red stained calcisphere15
(shown right) is interesting with its outer pale green layer.
This suggests a pure calcite interior with an outer layer of
ferroan dolomite. The slow change of calcite (diagenesis) to
dolomite has occurred in a number of stone bands in the Dorset
Kimmeridge Clay. This may have biological origins. The further
conversion to ferroan dolomite suggests some anaerobic conditions
in this particular case, since minute quantities of the reduced
form of iron (II) appear to have displaced calcium in the
carbonate rock, in the nannofossil. Elsewhere on this peel
calcispheres have been found showing a deep blue interior,
indicative of ferroan calcite. These are further evidence for
anaerobic conditions on a minute scale. The presence of fusain
and pyrites buried in the kerogen on Figure (3) suggests another
reason for the anaerobic environment.