|
|
A Gallery of Acetoacetanilide Photomicrographs (using
polarized light) |
|
|
A Gallery of Acetoacetanilide Photomicrographs (using
polarized light) |
Acetoacetanilide
is not a chemical that normally would be found in the household
environment. It is an "intermediate" used in the production of
organic dyes and pigments, (particularly yellow ones), and in the
formulation of some pesticides.
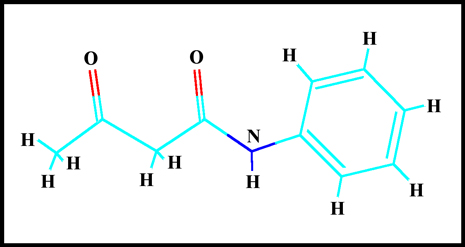
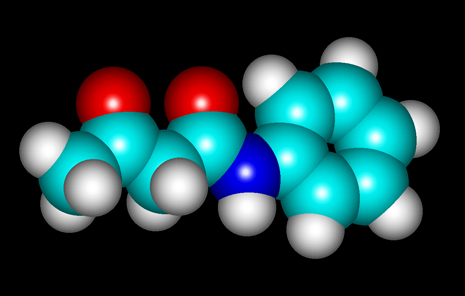
The molecular structure of the
compound can be seen below. "HyperChem" software was used to
visualize the molecule.
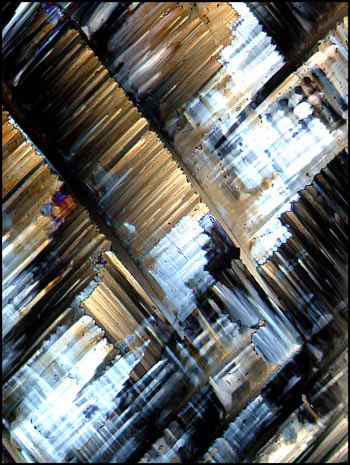
When viewed under the microscope
between crossed polars, acetoacetanilide often produces very colourful
fields that tend to look rather "flame-like". The two images
below were produced using a lambda/4 compensator in addition to polars
in their extinction position.


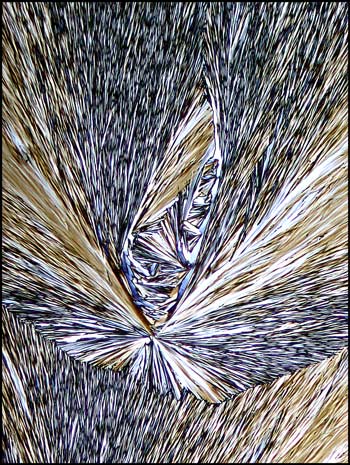
Without the compensator, the
colours appear darker and have greater contrast, as can be seen in the
example below.

As the compensator is rotated, the
colours of various sections of the field change. Notice how one
particular field looks completely different as the angle changes.


If, for some reason, the layer of
crystals between slide and cover-slip changes in thickness, (for
example if the cover-slip is not parallel to the slide), completely
different formations may appear in thinner regions. The colours
in thinner areas are less brilliant and tend towards gray. The
image on the left shows a field from a "thicker" region, and the one on
the right from a "thinner" one.

The colours however, can be
determined by other factors. Immediately after the melt
re-solidifies, large sections of the slide have the following
appearance.
Within minutes however, colourful
fan-shaped formations seem to grow into the darker areas. Notice
the process occurring in the top right corner of the two images
below. The right image was photographed about thirty seconds
after the left one. I believe that as the second (colourful) form
grows, it is actually converting the molecular packing type from one
form to another. In the end there is only one molecular
arrangement.


After this process has completed,
the fields look like the one below. Both images were produced
with crossed polars, but the one on the left used two lambda/4
compensators, while the one on the right used lambda/4 and lambda
compensators.

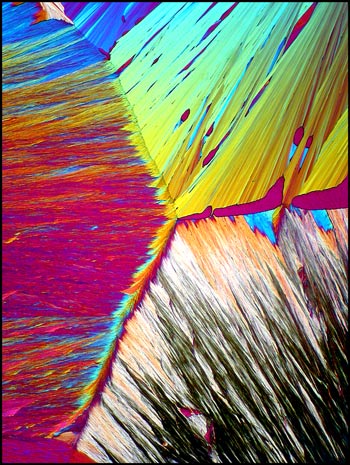
The image below shows a curious
formation right at the edge of the crystal melt (where the cover-glass
ends). The same compensators were used as in the image on the
right, above.
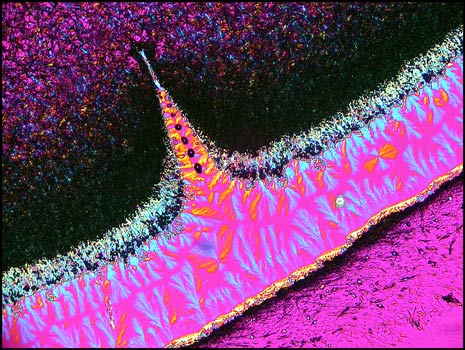
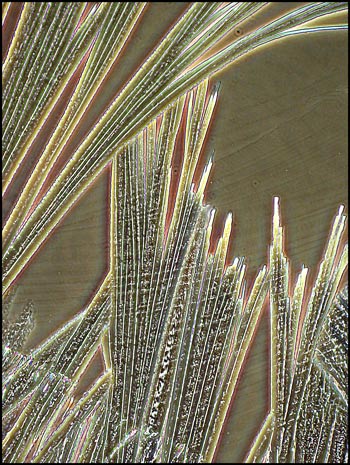
The hair-like formations below were
also observed right at the edge of the crystal melt. A polarizing
condenser with crossed polars and no compensators was used to produce
the image. Beneath it is another taken at a higher magnification
utilizing a phase contrast condenser to show the detail.

Acetoacetanilide certainly produces
a wide range of interesting crystal formations. A single attempt
at producing a melt specimen can provide a rewarding evening of study
and many potential photomicrographic subjects!
The photomicrographs in the article
were taken with a Nikon Coolpix 4500 connected to a Leitz SM-Pol
microscope.
Published in the March
2006 edition of Micscape.
Please report any Web problems or
offer general comments to the Micscape
Editor.
Micscape is the on-line monthly magazine
of the Microscopy UK web
site at Microscopy-UK
© Onview.net Ltd, Microscopy-UK, and all contributors 1996 onwards. All rights reserved. Main site is at www.microscopy-uk.org.uk with full mirror at www.microscopy-uk.net .