Topical
tips 15: Using aquarist test kits for simple analysis of 'pond dipping' samples.
Plus
brief notes on electronic pH and conductivity meters.
by David Walker, UK
A
wander around larger pet supply stores will usually reveal a good variety of
test kits for the aquarist to check various properties of aquarium water.
These kits sell for a few pounds and are also useful for the microscopy enthusiast to assess some water
properties of
freshwater habitats studied.
A basic
appreciation of a water body's chemistry is one step
to put freshwater studies on a firmer ecological footing. In
the author's home area for example, stone water troughs are a favourite habitat to
study but it isn't always obvious whether a trough is filled from rainwater, a
spring,
run-off from roads, livestock fields or how it varies during the year. A few
simple analyses using these kits can offer some insight into these
factors.
Shown
below are a selection of the type of kits the author has tried and found useful.
The actual makers may vary depending on where bought. On-line dealers also offer
a wide range if no local large pet store. Only a brief note on the
value of each measurement type is made; the many books on limnology and freshwater
ecology can provide a good introduction. The author also offers
a few thoughts on some cheap electronic pH and conductivity test methods.
|
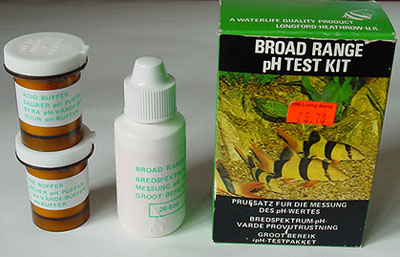 
pH: Many freshwater organisms have specific pH preferences
and tolerances.
pH
- Broad
range kits: This type of kit is my favourite and the one that gets
most use, more so than an electronic pH meter. Very often all I
wish to know is a rough idea of the acidity or alkalinity and this sort of
kit does it well with clear colours for each pH. It's compact and
very quick to do at pond/stream side to assess for this kit pH 4-10
to nearest unit. Fill the water to the 10 ml
level, add four drops of indicator and check colour against the bottom row
on box.
(The pots of chemicals shown aren't needed, these are buffers for
aquarists to adjust aquarium pH.) Maker 'Waterlife'. Indicator sufficient
for ca. 150 tests. The colour range for their narrow range kit pH
6.6-7.8 is along top of box.
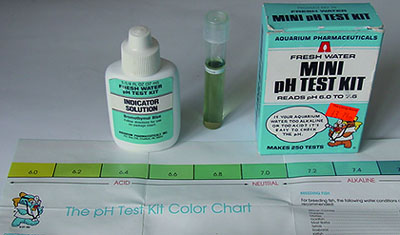
pH
- Narrow
range kits: These can narrow down the pH but only
perhaps to the nearest 0.4 - 0.6 rather than the optimistic 0.2 of the
scales as the colour changes can be subtle. If accuracy better than
ca. 0.5 is required this is where a pH meter
would be better. This type of kit can only be used of course when
it is known from a broader measurement that the pH lies in the kit's
range. Maker 'Aquarium Pharmaceuticals'. pH 6.0 - 7.6. 250 tests.
ca. £4.
In
the author's opinion the above two aqueous kits give easier to interpret
colours than the rolls of broad / narrow range pH paper familiar
from school labs. The colours often seem to fade on these papers
with storage and colours weak.
Although there are modern small plasticised strips with bonded indicators for
pH measurement and a wide range of other tests, but these aren't always available
outside of the lab. suppliers and can be expensive.
|
|
  
Water
hardness: Some
organisms require
calcium for their shells and exoskeletons.
The
kit above is an example of the colorometric tests for either total
water hardness (calcium and magnesium) or calcium. The number
of drops of the final reagent required to change the colour of the
sample from red to blue are counted. The number of drops x10 gives,
in this case total hardness, in ppm. This kit and nitrate one
below are more expensive than pH kits at ca. £7 but would probably
be used less regularly for a given water body. Maker of above
kit 'Aquarium Pharmaceuticals'.
|
|
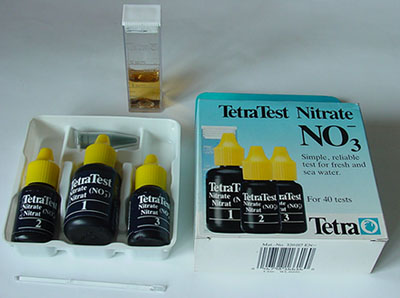 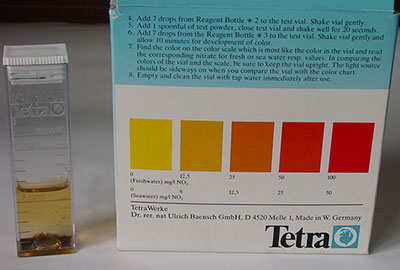
Nitrate:
Nitrate is the end product of the aerobic degradation of organic
and inorganic nitrogen compounds present in water. Fertilizer run
off from surrounding land can also influence the nitrate levels.
The nitrate content can provide an indication of the level of pollution,
which in turn will affect the occurrence of organisms sensitive
to or tolerant of such pollution levels. In general, nitrate concentrations
from 25 - 100 mg / l or more indicate increasing levels of pollution.
The
nitrate test kit above is a typical maker's example with three reagents and pot of 'powder' with spatula.
After completing the test sequence the colour after 10 minutes is
compared with that on scale on the box for nitrate concentration.
Maker 'TetraWerke' ca £9-50 for 50 tests.
|
Electronic
methods for pH and conductivity
The
cost of pocketable pH meters and conductivity meters has plummeted in recent
years and are widely available. A multi-purpose meter may be worth considering,
some now measure temp, pH and conductivity.
It
is vital that meters are also bought with the recommended calibration solutions
and, for pH measurements especially to be worthwhile, the user must be prepared to adopt a more rigorous approach,
with initial and regular calibration, correct storage, standardised sample measurement
etc. Metrohm, one of the leading
makers of such equipment, are admirably succint with their opinion of
pH measurements without calibration (see first link in resources below). It's worth keeping a
calibration record sheet for each meter.
|
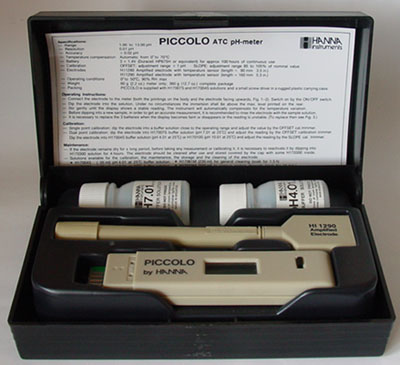 Electronic
pH meters: It's
worth comparing specs of models before purchase; some of the cheapest
may have no temperature compensation or may resolve to two decimal places
when its accuracy is 0.2. The model shown does have an accuracy
of
0.02 but on reflection was overkill for casual studies, a one decimal
place model would have been sufficient. The
cheaper kits don't always come supplied with two calibration solutions as here
(although these small pots will require replacement). I haven't
found this design as practical as the shorter more pocketable designs. Electronic
pH meters: It's
worth comparing specs of models before purchase; some of the cheapest
may have no temperature compensation or may resolve to two decimal places
when its accuracy is 0.2. The model shown does have an accuracy
of
0.02 but on reflection was overkill for casual studies, a one decimal
place model would have been sufficient. The
cheaper kits don't always come supplied with two calibration solutions as here
(although these small pots will require replacement). I haven't
found this design as practical as the shorter more pocketable designs.
Errors
are more likely to creep into the often more casual and occasional studies
the enthusiast may make cf. a lab's studies where an electrode will be correctly
stored and invariably the calibration checked before each study. The hobbyist's meters may be left for some time and
less than ideally
stored. To demonstrate the potential inaccuracies, I measured a known calibration
solution after a few months storage without performing the maker's
recommended 4h reactivation procedure. The stabilised reading was 0.4
too low.
|
|
 Conductivity:
The electrical conductivity of water provides a measure of the
total concentration of dissolved ions present and hence provides
a general indication of the inorganic nutrient concentration of
a water body. Conductivity:
The electrical conductivity of water provides a measure of the
total concentration of dissolved ions present and hence provides
a general indication of the inorganic nutrient concentration of
a water body.
The meter
right is a typical model and very portable. Such meters, as did the author's,
do not always come with a calibration solution and may give the
impression that they are accurate straight out of the box. Not necessarily
so, if the author's example is typical as it was reading 20%
low before calibration.
Unlike
pH meters, however, which typically have thin glass membranes which
can change property with age and storage, the conductivity meters
are measuring conductivity between two metal probes so are
a lot more robust and less likely to change. After the initial
calibration of the author's example it has remained fairly accurate
with only occasional need for adjustment over nine years of
intermittent use and storage.
A conductivity
measurement of an unfamiliar water body is useful before pH
measurements as it can show if it's of low ionic strength and thus potentially 'weakly buffered'.
For such samples the pH can shift just by the act of measuring the pH
so for more detailed projects studying such water bodies needs particular care in choice
of equipment and measurement (as do accurate pH measurements of rainwater).
A temperature
compensated meter is worthwhile as conductivity is strongly temperature
dependent. An auto shut off model is also useful. Waters with
high salinity may need a meter with higher range.
Make / model shown, Hanna DIST WP3, range 0-1990 µS/cm. Resolution
10µS, automatic temp. compensation. ca £53 nine years ago, cheaper
nowadays.
|
Comments to the
author
David
Walker
are welcomed.
On-line
resources - a selection:
Metrohm's
downloadable 'The
background of pH measurement and hints for your daily work'
(12 pages). Note section 3.4.1 on the validity of pH measurement without calibration!
Radiometer
Analytical's MeterLab Documentation
web page has superb free downloadable pdf articles on 'pH Theory and practice
guide' (34 pages) and 'Conductivity theory and practice guide' (54 pages).
'Water
pH' an
article on the US
Fish and Wildlife Service
website is a useful overview of theory and practice of the pros, cons of different
water analysis methods.
Metrohm's
guidelines and recommended meters for pH measurement of water samples with low
conductivity (defined by them as <200 µS/cm) and high conductivity.
An
interesting thread on ChemTrail
Central
reporting and discussing the relative pros and cons of techniques for measuring
the pH of rain water.
Water
analysis equipment is one of a number of items for a microscopist's laboratory.
Richard Howey gives a valuable discussion in his four part series 'Equipping
a laboratory'.
Suppliers:
An 'aquarist
"test kits"' search in Google shows many online dealers. Electronic
meter makers include Hanna
Instruments who have a very wide range. The 'Checker' model sells frequently
on UK eBay for <£20, but don't forget the calibration solutions.
© Microscopy UK or their contributors.
Published in the March 2005 edition of
Micscape.
Please report any Web problems or offer general comments to
the
Micscape
Editor
.
Micscape is the on-line monthly magazine of the Microscopy
UK web site at
Microscopy-UK
© Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is
at www.microscopy-uk.org.uk
with full mirror
at www.microscopy-uk.net
.








 Electronic
pH meters: It's
worth comparing specs of models before purchase; some of the cheapest
may have no temperature compensation or may resolve to two decimal places
when its accuracy is 0.2. The model shown does have an accuracy
of
0.02 but on reflection was overkill for casual studies, a one decimal
place model would have been sufficient. The
cheaper kits don't always come supplied with two calibration solutions as here
(although these small pots will require replacement). I haven't
found this design as practical as the shorter more pocketable designs.
Electronic
pH meters: It's
worth comparing specs of models before purchase; some of the cheapest
may have no temperature compensation or may resolve to two decimal places
when its accuracy is 0.2. The model shown does have an accuracy
of
0.02 but on reflection was overkill for casual studies, a one decimal
place model would have been sufficient. The
cheaper kits don't always come supplied with two calibration solutions as here
(although these small pots will require replacement). I haven't
found this design as practical as the shorter more pocketable designs.
 Conductivity:
The electrical conductivity of water provides a measure of the
total concentration of dissolved ions present and hence provides
a general indication of the inorganic nutrient concentration of
a water body.
Conductivity:
The electrical conductivity of water provides a measure of the
total concentration of dissolved ions present and hence provides
a general indication of the inorganic nutrient concentration of
a water body.