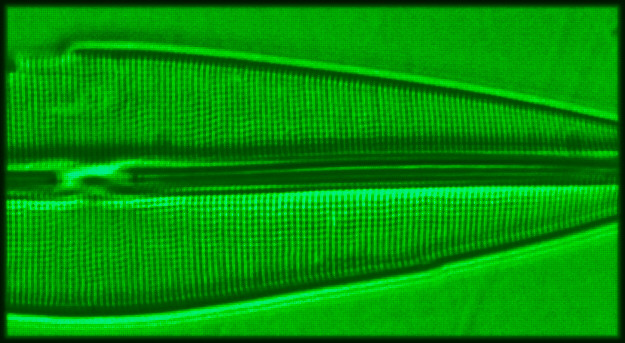
|
I compared BF, BF with a green interference
filter, COL, DF, and DF with a blue filter using
images taken from Stauroneis phoenicenteron. I
used an achromat 40x objective with NA 0.65 for
all DF work to ensure that my simple darkfield
stop in my Abbe slider condenser offers optimal
DF. I used a semi-apochromat 40x objective with NA
0.75 for BF and COL. Since the Abbe condenser
offers an NA of around 0.75 when used dry, my
setup for COL does not contain much light from the
so-called DF component, which is light that is
only captured by the objective when diffracted in
the specimen plane. The results are depicted in
Figure 2. When taking these photomicrographs, I
carefully focused on just one row of dots located
towards the middle of this diatom.
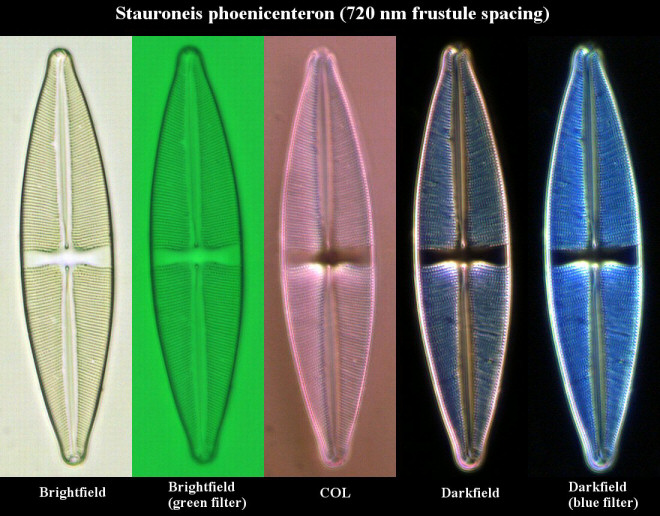
|
|
|
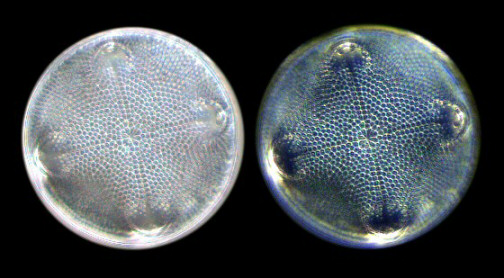
Figure 2 - Diatom Stauroneis
phoenicenteron. For the first three
images, I used a semi-apochromat 40x
objective with an NA of 0.75. For the last
two images (darkfield images), I used an
achromat 40x objective with an NA of just
0.65. The images were recorded with a
digital camera using a CMOS image sensor.
(Click on image for larger
version.)
|
In a second step, I cropped the row I selected for
focusing, including some neighboring rows, out of
each of the five individual images and enlarged
the cropped sections by a factor of two for better
visibility. I summarized the results in Figure 3.
For reference, I also added a higher resolution
image that I obtained with an achromat 100x
objective with NA 1.25 using BF (see lower right
image in Figure 3). Of course, I used immersion
oil for this reference image. Figure 4 shows
Stauroneis phoenicenteron observed with an
achromat oil immersion 100x objective and an
achromat condenser with NA 0.90 using BF. For
Figure 4, I did not use immersion oil between the
achromat condenser and the slide. Of course,
Figure 4 does not show a real high-resolution
image of this diatom. Using a special darkfield
oil immersion condenser, finer detail could be
revealed.
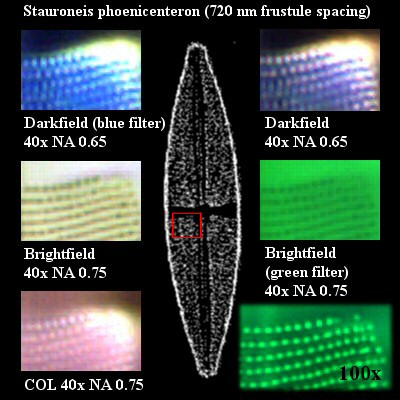
|
|
|
Figure 3 - Five of these image sections
are taken from the images of Figure 2. The
inset depicts a diatom with a red square
surrounding the area that has been cropped
out. The image, which is located towards
the bottom right, shows the same section
of this diatom recorded with an achromat
100x oil immersion objective with an NA of
1.25.
|
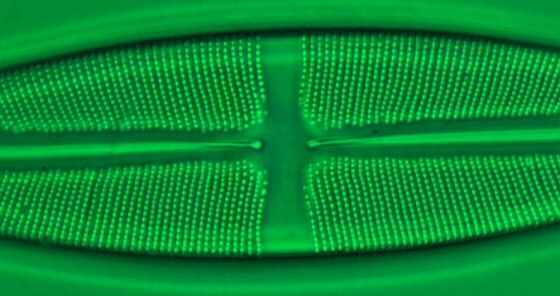
|
|
|
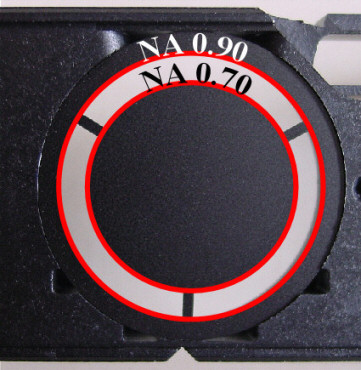
Figure 4 - For this image of Stauroneis
phoenicenteron I used an achromat 100x oil
immersion objective with an NA of 1.25
together with an achromat condenser with
an NA of 0.90/dry using BF. A green
interference filter was used. (Click on
image for larger version.)
|
I was using a PixeLink PL-A662 FireWire digital
camera. This device offers great flexibility to
control the imaging process and connects directly
to a C-mount adapter connected to a trinocular
viewing body. When looking at these images of
Figure 3 that were obtained with DF, we can easily
see very bright sections indicating intensities
that were not adequately captured by the digital
imaging device (see upper right corners of the two
topmost images in Figure 3). This has nothing to
do with DF but is due to a limitation of the
10-bit sampling used by the digital imaging
device. Today, most digital imaging sensors
perform the analog to digital (A/D) conversion at
a 12-bit or even 14-bit sampling rate.
DF is indeed a very powerful method to study the
fine structure of diatoms. DF illumination is easy
to setup and, a little to my surprise, I
recognized more detail in this particular
structure using DF together with the cheaper 40x
objective than I was able to see with BF or COL
using the much more expensive semi-apochromat.
|