CRICKET
EMBRYOS IN 3D
A brief contribution to 3D photography
by Robert Sturm, Salzburg (Austria)
As I could
demonstrate in a contribution previously published in Micscape, embryogenesis of Orthopteran insects such as grasshoppers
or crickets is commonly characterized by a multiplicity of developmental
stages. As shown in the sketch below, embryonic development of the Orthoptera
(in the concrete case: Locusta migratoria)
commonly starts with the fertilized oocyte (= egg cell), which undergoes an
extensive process of cell division. This procedure results in the production of
a multiplicity of new cells, which subsequently grow and differentiate into
those organs needed for the next developmental stage. The embryogenesis of
insects represents a specificity insofar as early egg cleavage only involves
nuclear subdivisions, which are not accompanied by respective partitions of the
cytoplasm. This phenomenon is commonly known as so-called syncytial or superficial cleavage. The first terminus is derived from the fact that cells containing a
high number of nuclei form a syncytium. The second terminus indicates the fact
that this specific development mainly takes place near the surface of the egg
cell.
After extensive formation of several thousand cell
nuclei, these cellular compartments are subjected to a migration process,
during which they move towards the egg periphery and produce a layer containing
a multitude of cell-like structures (energids). Among scientists this layer is
known as blastoderm, which itself
may be subdivided into the germ band or ventral plate, the initial stage of the embryonic body, and the serosa forming the yolk sac. The germ band defines the
origin of the embryonic body and is subsequently subjected to an extensive
process of differentiation, which results in the mapping out of the fundamental
body plan of the insect. The germ band undergoes a continuous enlargement, in
the course of which the three germ layers (endoderm, mesoderm, and ectoderm) are formed.
From these histological units different insect organs emerge during the
essential processes of histogenesis and organogenesis. After completion of tissue and organ development,
the differentiated embryo undergoes a procedure of intense stretching, muscle contraction,
and uptake of gas into the tracheoles. The final stage of embryonic development
is characterized by the hatching process, where the animal comes out of the
egg.
If we take a closer look to hemimetabolous insects,
where a nymph stage is intercalated
between embryonic and adult phase, all developmental stages described above are
significantly marked by a process called blastokinesis. Here, the organism is
subjected to rotational and translational movement during its growth. As
illustrated in the figure at the left, blastokinesis of the migratory locust
distinguishes itself by a displacement of the embryonic head from the back pole
to the front pole of the egg. In other words, the embryo executes a 180°
rotation within its very limited space. In the case of the house cricket and
the field crickets (e.g., black field cricket) the germ band has already the
right orientation, and blastokinesis takes a complex course with temporary
submersion of the embryo into the yolk sac.
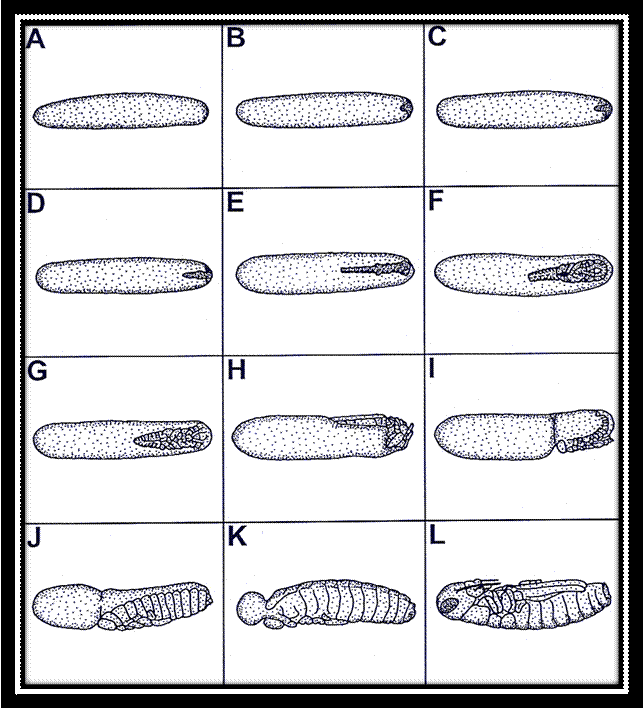
Single
stages of the embryonic development of the migratory locust Locusta migratoria: A-C: superficial
cleavage and development of the blastoderm, D-F: development of the germ band
and germ layers, G-I: development of the fundamental body plan and
blastokinesis, J-L: re-orientation of the embryo, development of the amnion,
and final differentiation of the body.
Photography of cricket embryos has to be regarded as a
special challenge in several respects: In the normal case the egg chorion has no
transparency at all, so that under the light microscope only the surface of the
egg can be studied, but not its internal structures. In order to overcome this
enormous deficit, a special fixation technique has to be applied, which was
originally developed by Wolfgang Groepler in the 1980s and produces a partly
transparent chorion. Another problem is given by the circumstance that duration
of cricket embryogenesis depends on a multitude of external factors, among
which environmental temperature plays a superior role. This means that single
developmental stages such as those shown in the illustration above are only
accessible under controlled laboratory conditions. (For a detailed description
the reader is kindly referred to my papers listed below.) Only by knowing the
environmental conditions and the related velocity of embryonic development a
complete sequence including all embryonic stages can be sampled from the
incubated eggs.
3D
photography of selected embryonic stages (animated images)
After all the theory
noted above I have selected four stages of the embryogenesis occurring in the
house cricket (Acheta domesticus).
From these stages I have produced 3D photographs. I have decided to present the
respected images to the interested reader in two different ways: (1) as
animated GIFs and (2) as classical anaglyphs that have to be watched with
red-green glasses. Animated GIFs generate a three-dimensional effect of the
objects by simply simulating their rotation by an angle of several degrees.
This can be among other achieved with the help of specific computer software
such as PICOLAY developed by
Heribert Cypionka. After import of a selected photograph into the working area
of the program the user has to export the image to the results window and has
to generate a depth map of the recorded object. This step is of immense
importance, because based upon this depth map, where weakly illuminated parts
are assumed to define the background, whilst highly illuminated parts are more
probably placed in the foreground, a stack of images used for the production of
the three-dimensional picture is computed. The animated GIF resulting from this
procedure can be manipulated in several ways (e.g., change of the rotation
angle, modification of the rotation direction), so that it needs some time to
obtain optimal results.

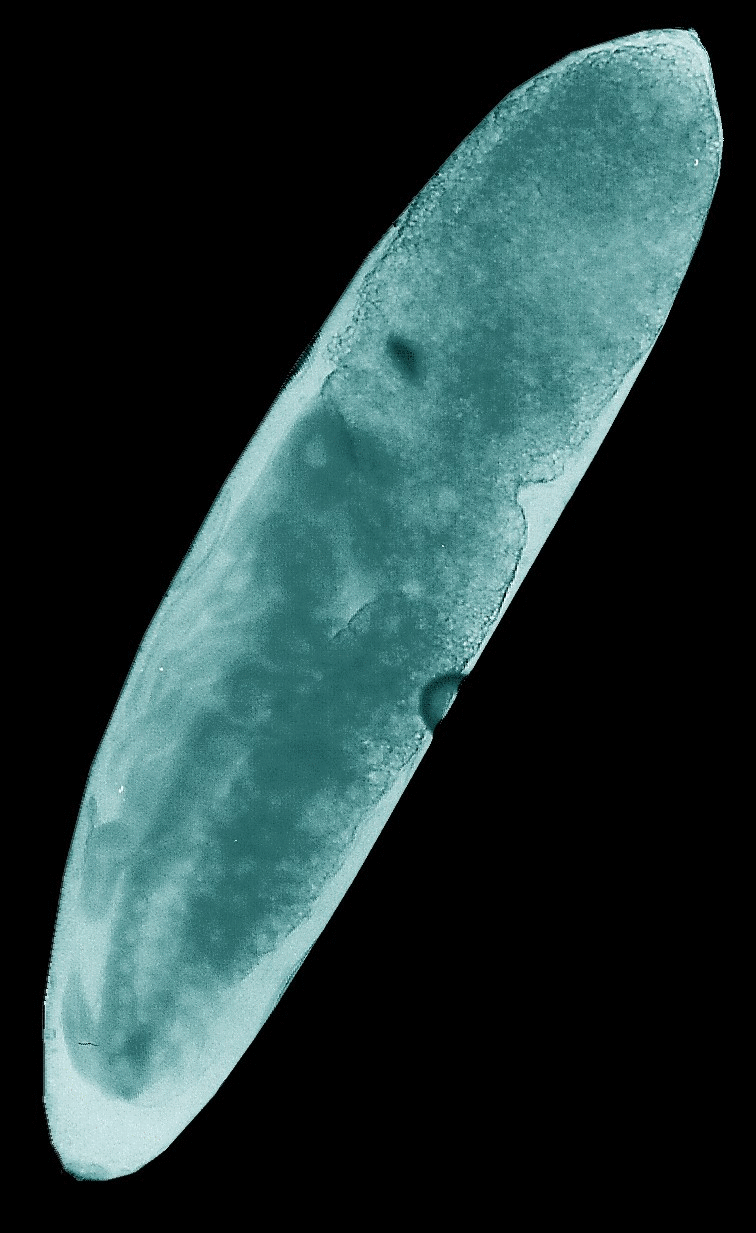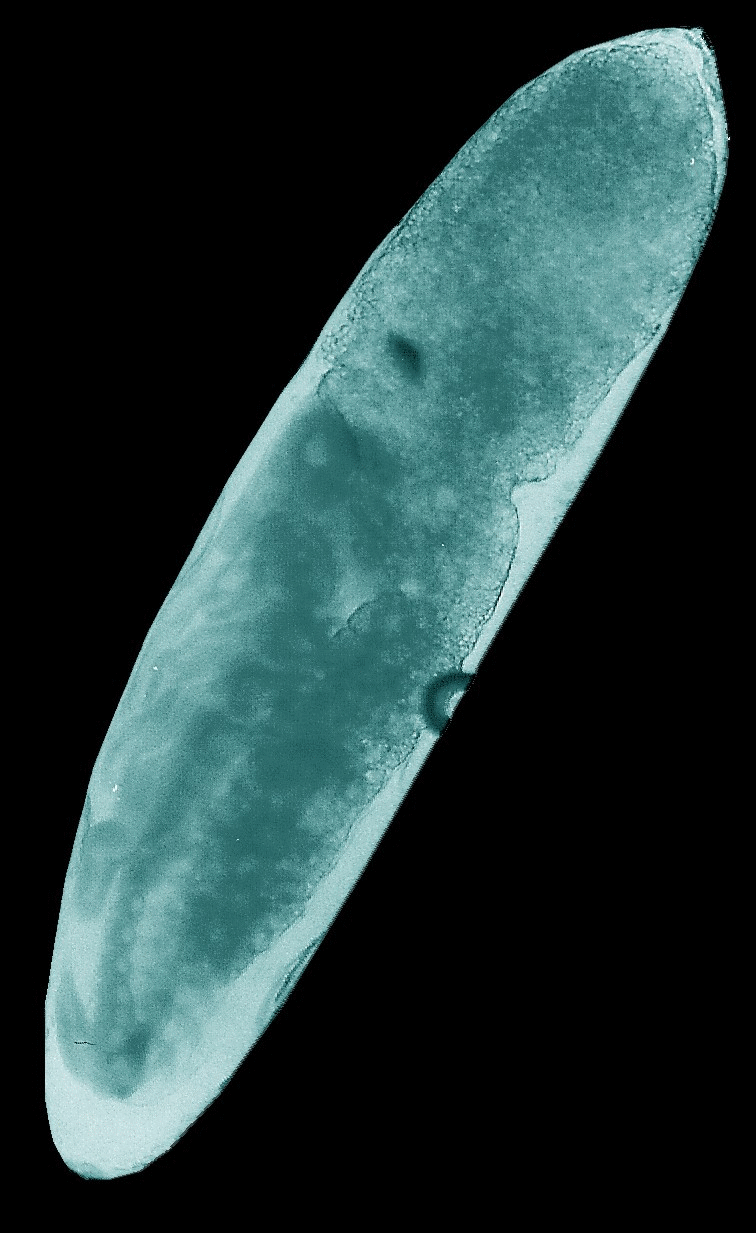


Animated
GIFs of four embryonic stages occurring in the house cricket Acheta domesticus. Upper left: fertilized,
largely undifferentiated egg with its homogeneous yolk mass, Upper right: stage
J illustrated in the figure above with progressed organization of the thoracic
extremities and initial segmentation of the abdomen. Lower left: late stage of
embryogenesis (K-L) with completed head, body segments and extremities, but
uncompleted back. Lower right: fully differentiated embryo ready to undergo the
hatching process.
3D
photography of selected embryonic stages (red-green anaglyphs)
At the end of
my brief contribution I also want to present red-green anaglyphs of the four
embryonic stages described above. In order to obtain the three-dimensional
effect the use of red-green glasses, which are available in the World Wide Web
or can be produced by oneself after purchase of appropriate colour films, is
necessary. With respect to animated GIFs anaglyphs bear the huge advantage that
they can be also watched aside of the computer and thus can be used in printed
form.


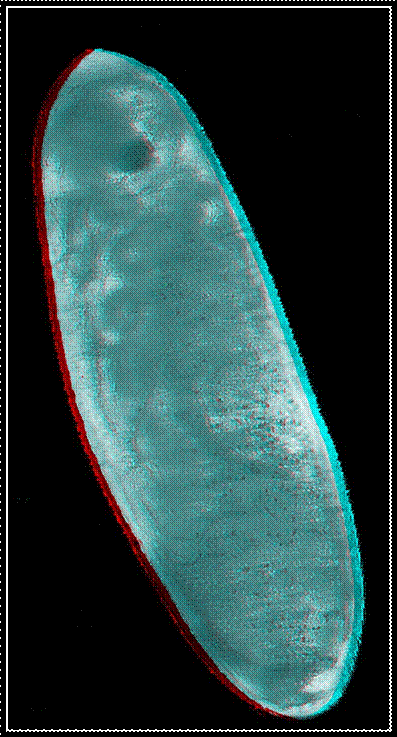
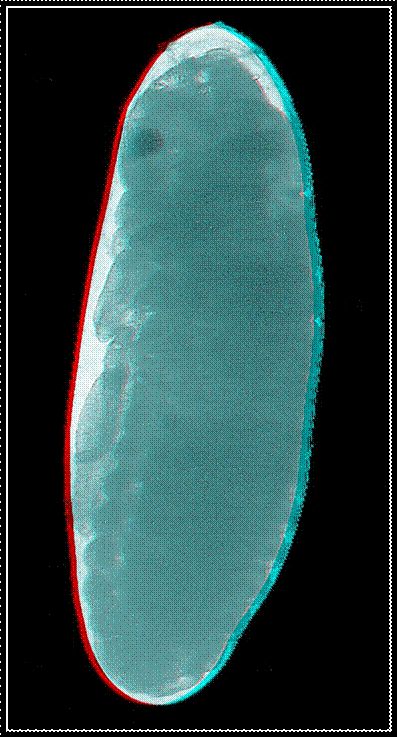
Red-green
anaglyphs of four embryonic stages occurring in the house cricket Acheta domesticus. For an appropriate
perception of the three-dimensional effect the use of red-green glasses is
necessary.
For all those readers, who are interested in rearing,
growth and development of various cricket species, I have listed some of my
scientific works published hitherto. You can find free copies of most of them
on my Research Gate website.
1. Sturm,
R. (2006). Vom Ei zum Adulttier - Mikroskopische Dokumentation der Keimes und
Jugendentwicklung bei ausgewählten Grillenarten. Mikrokosmos, 95, 305–309.
2.
Sturm,
R. (2016). Modeling larval growth of various cricket species. Mathematical
and Computational Biology, 5, 6.
3. Sturm,
R. (2016). Mathematische Modelle in der Biologie. Naturwissenschaftliche
Rundschau, 69, 500-504.
4. Sturm,
R. (1999). Einfluß der Temperatur auf die Eibildung und Entwicklung von Acheta domesticus (L.) (Insecta:
Orthoptera: Gryllidae). Linzer biologische Beiträge, 31(2), 731–737.
5. Sturm,
R. (2002). Einfluss der Temperatur auf die Embryonal- und Larvalentwicklung bei
verschiedenen Grillenarten (Insecta: Orthoptera). Linzer biologische Beiträge,
34(1), 485–502.
6.
Sturm, R. (2003). Längen- und
Gewichtsentwicklung der Larven verschiedener Grillenarten (Orthoptera:
Gryllidae) vom Zeitpunkt des Ausschlüpfens bis zur Adulthäutung.
Linzer biologische Beiträge, 35(1), 487–498.
7. Sturm,
R. (2008). Eiproduktion und Oviposition bei der australischen Feldgrille Teleogryllus commodus Walker, 1869:
Experimentelle Ergebnisse und Modellrechnungen (Orthoptera: Ensifera,
Gryllidae). Entomologische
Zeitschrift 118: 41-45.
8.
Sturm,
R. & Pohlhammer, K. (2000). Morphology and development of the female
accessory sex glands in the cricket Teleogryllus
commodus (Saltatoria: Ensifera: Gryllidae). Invertebrate Reproduction
& Development, 38, 13–21.
9. Sturm, R. (2002). Development of the accessory
glands in the genital tract of female Teleogryllus
commodus WALKER (Insecta, Orthoptera). Arthropod Structure &
Development, 31, 231–241.
10. Sturm, R. (2002). Morphology and ultrastructure of
the female accessory sex glands in various crickets (Orthoptera, Saltatoria,
Gryllidae). Deutsche entomologische Zeitschrift, 49, 185–195.
11.
Sturm,
R. (2005). Motoric activity of the receptacular complex in the cricket Teleogryllus commodus (Insecta:
Orthoptera: Gryllidae). Entomologische Abhandlungen, 62, 185–192.
12.
Sturm,
R. (2008). Morphology and histology of the ductus receptaculi and accessory
glands in the reproductive tract of the female cricket, Teleogryllus commodus. Journal of Insect Science, 8,
1–11.
13. Sturm, R. (2009). Morphology and histology of the
reproductive system in females of the black field cricket Teleogryllus commodus Walker 1869 (Insecta: Orthoptera): a drawing
study. Linzer
biologische Beiträge, 41, 863–879.
14. Sturm, R. (2012). Morphology and ultrastructure of
the accessory glands in the female genital tract of the house cricket, Acheta domesticus. Journal
of Insect Science, 12, 1–11.
15. Sturm, R. (2016). Morphology and development of the accessory glands
in various cricket species. Arthropod Structure & Development, 45,
585–593.
16. Sturm,
R. (2016). Studie eines Insektenorgans mithilfe unterschiedlicher licht- und
elektronenmikroskopischer Verfahren. Mikroskopie, 3, 209–219.
17. Sturm, R. (2003). The spermatophore of the black
field cricket Teleogryllus commodus
(Insecta: Orthoptera: Gryllidae): size, structure, and formation. Entomologische
Abhandlungen, 61, 227–232.
18.
Sturm,
R. (2011). The effect of remating on sperm number in the spermatophores of Teleogryllus commodus (Gryllidae).
Invertebrate Biology, 130, 362–367.
19. Sturm, R. (2014). Comparison of sperm number,
spermatophore size, and body size in four cricket species. Journal of
Orthoptera Research, 23, 39–47.
20. Sturm,
R. (2010). Experimente zur Nymphenentwicklung der australischen Feldgrille Teleogryllus commodus Walker 1869
(Insecta, Orthoptera). Articulata, 25, 45–57.
21. Sturm,
R. (2006). Computermodell zur Simulation der Eiablage des Heimchens Acheta domesticus (L., 1758). Articulata
21, 25–34.
22.
Sturm,
R. (2010). Life time egg production in females of the cricket Teleogryllus commodus Walker 1869
(Insecta: Orthoptera): Experimental results and theoretical predictions. Linzer
biologische Beiträge, 42, 803–815.
23. Sturm, R. (2015) Computer models in entomology:
Predicting the daily fecundity of female Acheta
domesticus. Mathematical and Computational Biology, 4, 5.
24. Sturm, R. (2016). Relationship between body size and
reproductive capacity in females of the black field cricket (Orthoptera,
Gryllidae). Linzer biologische Beiträge, 48, 1823–1834.
All comments to the author Robert Sturm are welcomed.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
© Microscopy UK or their contributors.
Published in the June 2017 edition of Micscape Magazine.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk .