|
Micscape Lite - contributors sharing general articles on topics other than microscopy. by David Walker, UK |
'Mindfulness' is a topic that seems very much in vogue nowadays. In recent years, it has been particularly associated with adult colouring books and in June 2015 a Daily Telegraph article noted that five of the top ten best selling books on Amazon were in this genre. My brother Ian has gained great pleasure in using them, creating some very attractive artworks. A browse around online resources reveal other practical activities associated with mindfulness including knitting, crochet, origami, jigsaws and carpentry. As an admittedly casual observer, the physical activities seem variously associated with bringing a feeling of well being, relieving stress and tension, aiding relaxation and a commonly used phrase—helping to achieve a state of 'being in the moment'.
I've recently shared on Micscape Lite aspects of my interest in building molecular models, both a review of five major modelling kits and two 'Molecules in the News' pieces including models of the six molecules that competed in the recent Nanocar Race held at CNRS in France. When building molecular models, especially the more extensive structures that can take some time to construct, I found it very relaxing with a sense of achievement on completion. There does seem to be a number of parallels building such models with the craft activities associated with mindfulness, so I explore briefly these parallels below—perhaps slightly tongue in cheek but there may be a nugget of sense somewhere and you don't need to have a chemistry background to enjoy making many of them! For budding molecule builders where any mention of chemistry causes glazed eyes, many illustrated can equally be regarded as geometric structures and can be appreciated on that basis. Illustrated with examples of molecular models from Molecular Visions, Minit dedicated kits.
Exploring the parallels, with examples.
A physical activity using the hands
An obvious point perhaps, but for me at least, making physical models distinguishes it from the software equivalent of the practice. Although creating molecular models on screen can be very satisfying, it is one step removed from making something that can be finished and physically handled. As the models become more extensive, using shortcuts will be tempting in software e.g. to cut and paste repeating structures or, counter productive (in the mindfulness context), downloading the already constructed
model from a database. There's no short cuts with physical models!
Starting with simple 'raw materials'
Craft activities would start with appropriate raw materials whether coloured pens with drawing book, balls of wool, thread, sheets of paper or pieces of wood. Making a molecular model would start with the atom centres and bond links of a particular kit. As remarked it can be the more extensive models that can engender the feeling of mindfulness and it is these type of molecules which are often available as kits for that structure so all the exact type and number of parts are supplied. Examples
include ice, buckminsterfullerene, nanocarbon structures, protein alpha helix, sodium chloride, other ionic solids, and other carbon allotropes.
A sense of progress being made as construction continues over some time
With the craft activities such as colouring or knitting, there is a sense of progress to maintain the interest and attention as the final product slowly takes shape. The same applies to molecular models with more extensive structures. Intermediate structures, the building blocks effectively can be constructed first as shown below for buckminsterfullerene, ice and a carbon nanotube.
Some basic skills required but involves repetitive actions that allow the mind to wander
Most craft activities associated with mindfulness require a minimum level of skills to make progress but also involve a high degree of repetitive activities with the hands. Making the molecules with more extended structures is very similar, the construction units of e.g. ice, carbon allotropes, are easy to make and assemble with instructions supplied by the kit maker.
A final product that engenders a sense of satisfaction on completion
As with craft activities, a final product is created and can be enjoyed and displayed. The more extensive structures shown can take some time to create so there's a sense of achievement when completed. Building such models admittedly doesn't have the same sense of creativity that crafts with an artistic aspect do, such as colouring. Although there's nothing to stop creation of other more fanciful structures. My brother Ian enjoys the symmetry of geometric structures and
built a Platonic solid the dodecadron. On checking, this molecule does exist as dodecahedrane, as do two other Platonic solids—tetrahedrane (only as substituted derivatives) and cubane. See the Wikipedia entry Platonic hydrocarbon.
Building such structures can also encourage an exploration of the scientific and technological background. Graphene and carbon nanotubes for example are being explored for a variety of roles because of their physical and electronic properties. The personalities associated with the first synthesis of some structures can also be explored. Three chemists who were associated with the first synthesis of buckminsterfullerene ('bucky-ball') were awarded the Nobel Prize for Chemistry in 1996 'for their discovery of fullerenes'.
Examples
All the examples other than ice are of carbon allotropes i.e. structures that pure carbon can form. We are all familiar with graphite and diamond from our school days but these are examples of much more recent forms that have been studied and synthesized. They have been in the news because of the potential exploitation of their often unique structural and/or electronic properties.
Graphene and carbon nanotubes

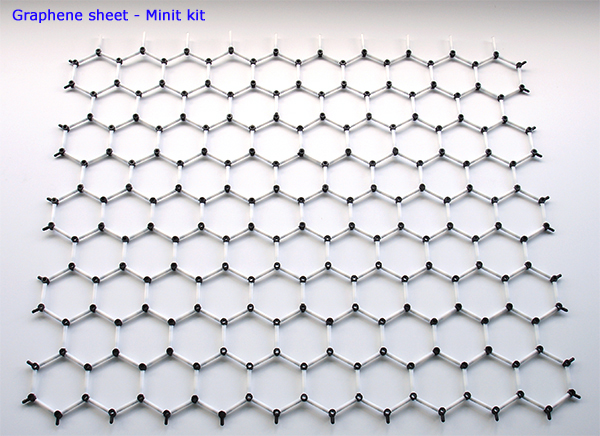
Above. A graphene sheet using the dedicated Minit kit by Cochranes of Oxford and available from their website. One of the simplest structures to build being a simple honeycomb type structure one carbon atom thick but a material of great interest to science and industry at present. It's also a precursor to some more striking 3D structures - carbon nanotubes. The sheet is rolled up and the upper row of bond links are attached to the lower line of free carbon links to make the chair form of a nanotube below. The structure uses solely the planar sp² carbon atom centres.
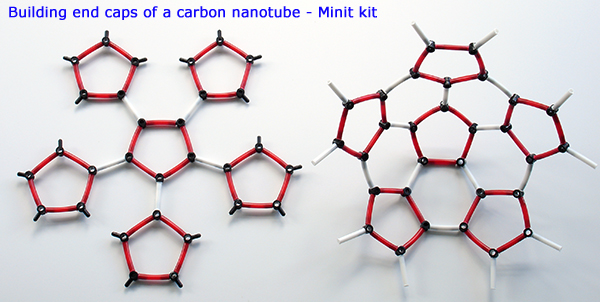
Above. The Minit kit also contains parts to form capped nanotubes. The end caps are essentially two halves of a buckminsterfullerene. The flat structure left is first built, then bonds between the five membered rigs pull it into a hemispherical form.
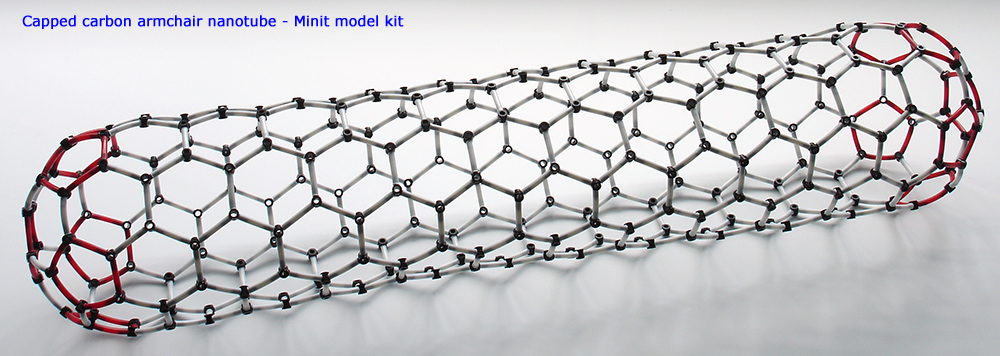
Above. A capped carbon nanotube using the graphene sheet and end caps built and shown earlier. This is a so-called chair form of nanotube. By starting with different arrangements of graphene sheet and how it is rolled up, two other forms can be made. The Minit leaflet supplied shows how. Notice that the carbon rings along the tube length are arranged linearly. Compare this to the tube shown later as part of a
nanobud.

Above. The leaflets supplied with the Minit graphene / nanotube / buckminsterfullerene kit and Molecular Visions 'Fullerene Model Kit' and 'Ice Crystal Kit' are easy to follow.

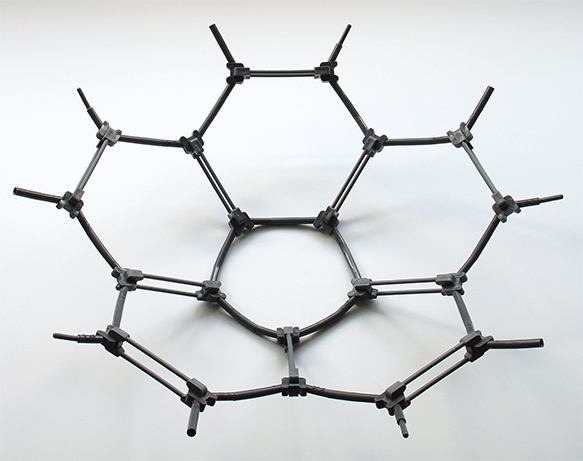
Above. Left. The Darling Models (Molecular Visions outside US) kit #7B to make the C60 buckminsterfullerene molecule is good value at $12. Simple instructions are provided. This kit includes the double bonds,
a simpler kit #7 at $9 omits them for an easier build. The builder starts with the parts bottom
left and can build the intermediate structures shown bottom right. This kit has easy to fit bond links and building the kit is very satisfying.
Right. A strong knowledge of chemistry isn't required to build these type of molecules and are often also mathematical / geometric interest as in this case—a truncated icosahedron. Building six membered rings around a central five membered ring, the builder can appreciate how the spherical structure is built up.
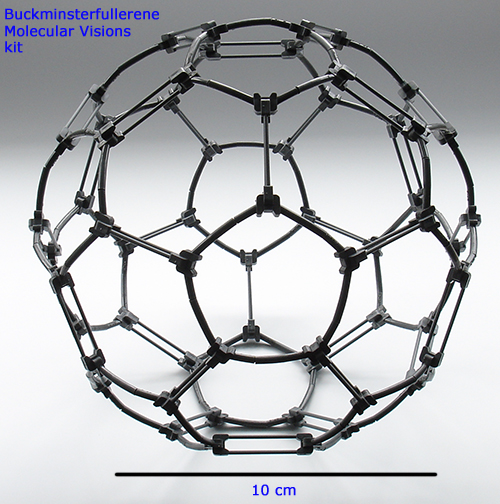
Above. The Molecular Visions completed model of buckminsterfullerene. This kit shows the double bonds.
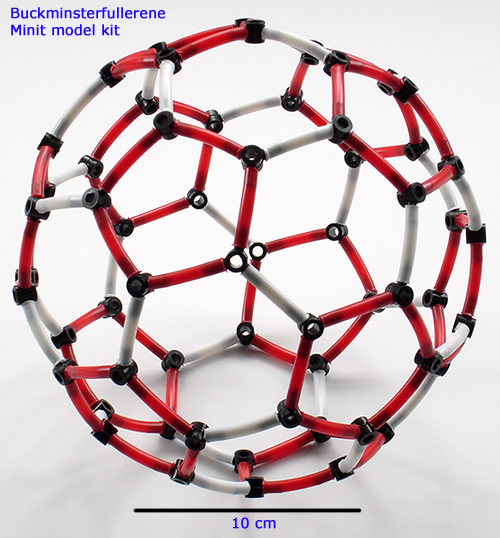
Above. As well as forming the end caps of the nanotube shown earlier, they can also be used to make buckminsterfullerene. The Minit kit adopts a two coloured linking system which the builder can use to illustrate features as desired. In this case, as shown above, to highlight the pentagon rings in the structure, rather than denoting double bonds. Double bonds are not favoured in the five membered rings (see Wikipedia entry).
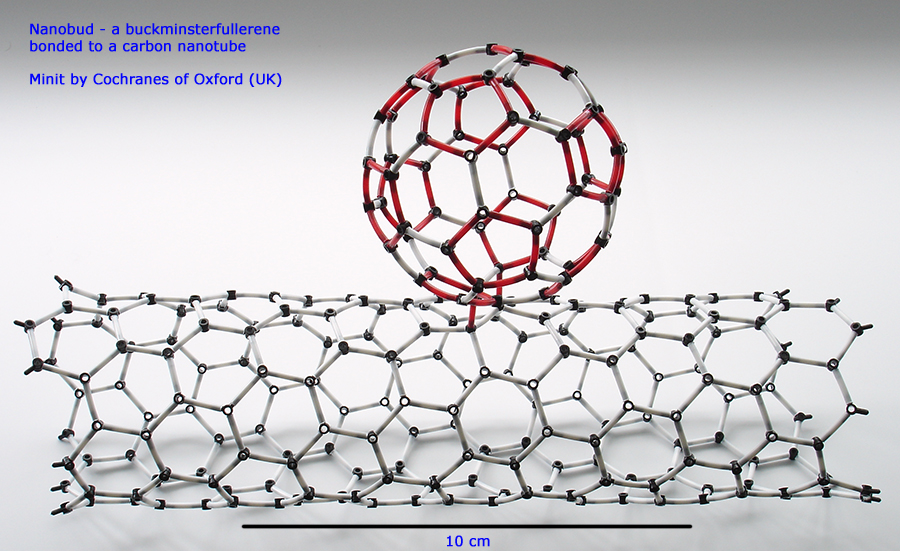
Above. This unlikely looking structure and others like it made solely of carbon have been successfully synthesized. They are great fun to make and a talking point if displayed! They are called nanobuds, in this example a Minit kit buckminsterfullerene is bonded to a Minit kit uncapped nanotube. The red links again are used to highlight the five membered rings, not the double bonds.
Although it looks complex, very little skill or any chemistry knowledge is required to make it from the Minit kit. The carbon nanotube shown is an alternative form that can be made in addition to the chair form shown earlier. In this form the carbon rings adopt a zig-zag structure along the tube length rather than a linear arrangement.

Above. A carbon nanobelt built using Molymod kit components. This has 12 edge sharing six membered phenyl rings and was reported by Itami et al in a paper in Science (14 April 2017) entitled 'Synthesis of a carbon nanobelt'. As the Abstract notes such a synthesis 'has been an elusive goal for more than 60 years'. Also see the public access article on the Phys.Org website dated 11 May 2017 'Synthesis of a carbon nanobelt with potential applications in nanotechnology'.
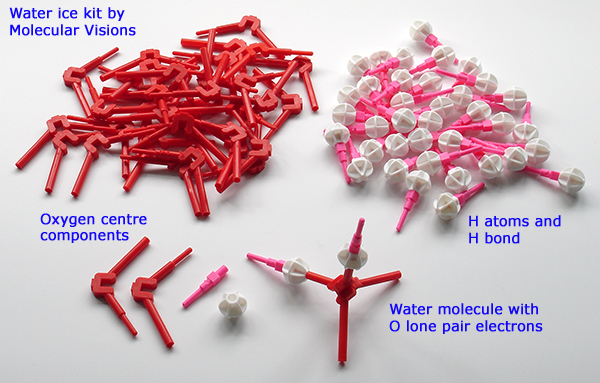
Above. The Molecular Visions kit #12 'Ice Crystal Kit' is good value at $12 and includes an easy to follow instruction leaflet. The final structure below can look complex, as can other solid state structures, but the simple stepwise approach to first build water molecules, adding the hydrogen bonds and building the cyclic sub-structures to then assemble the ice crystal does not require special skills or chemistry knowledge.
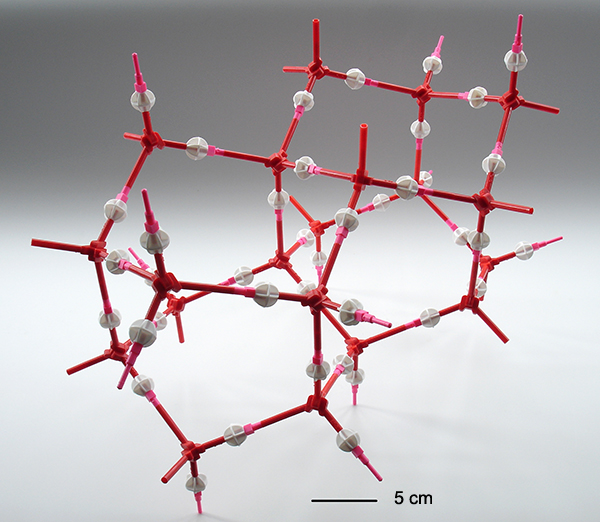
Above. A completed ice crystal model. The symmetry of this structure is especially appealing and instructive showing how the unique properties of water are associated with the hydrogen bonding between water molecules. A physical model clearly shows that the six membered rings of water molecules are in the 'chair' conformation in the horizontal planes and in the 'boat' form between the layers.
Comments to the author David Walker are welcomed.
Related Micscape Lite articles
A comparative review of five brands of molecular model kits. With notes on the educational merits of kits in an age of software. - compares five current major brands (ChemKits, Minit, Molecular Visions (Darling Models), Molymod, Orbit) with 'Molecules
in the News' in Spring 2017 supplement (heptacene, the 'elusive' triangulene and a molecular machine - a ratchet). April 2017 issue. PDFs
Molecules in the News II - illustrated with physical molecular models. Microscopical manipulation at the limit: The six molecules which participated in the first 'Nanocar Race' on 28-29th April 2017 organised by CEMES-CNRS,
France. May 2017 issue.
Published in the June 2017 edition of Micscape.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK web site at Microscopy-UK
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk.