
|
Rhapsody in C: Vitamin C (Part 1) by Richard L. Howey, Wyoming, USA |
Over the last several months, I have been absorbedmy wife says, fanatically obsessedwith taking photomicrographs of crystals under polarized light. It is, of course, inevitable that one develops favorites and a favorite of many, myself included, is Vitamin C (ascorbic acid) crystals, either by themselves or mixed with other substances. [CAUTION: As always, even with relatively harmless, common substances, be informed and careful when mixing them with other compounds. Don’t take unreasonable risks.] The results you get will depend, among other factors, on whether or not you are using a high grade, chemically refined version in crystalline form or whether you are using the powder from a crushed Vitamin C tablet that you purchased at your local drugstore. One might be inclined to think that Vitamin C is Vitamin C and that’s all there is to it. Well, not quite. I take a Vitamin C tablet every day as a supplement to maintain my general crankiness, since I have always been convinced that the “C” stands for “curmudgeon.” I have the bottle here in front of me on the kitchen table and the list of ingredients is as follows: Ascorbic Acid, Starch, Cellulose, Hydroxypropyl Methylcellulose, Crospovidone, Stearic Acid, Rose Hips, Magnesium Stearate, Silicon Dioxide, Hydroxypropyl Cellulose. The label also tells me that no preservatives or artificial colors have been added—wow, that’s a relief. Now, I suppose a pharmaceutical researcher would tell me that the Starch and Cellulose help hold the Vitamin C crystals (ascorbic acid) together in order to make a tablet and that Methyl Cellulose is Cellulose that’s inebriated on wood alcohol. Crospovidone is irritable povidone, which is a small Italian sausage. Stearic Acid and Magnesium Stearate are to enable you to drive your car better. Rose Hips? I didn’t even know they had legs! Silicon Dioxide—no wonder these tablets are so hard to swallow if they’re putting sand in them. And Hydroxypropyl Cellulose is, I suppose, Cellulose on steroids. Often labels distinguish between active and inactive ingredients, but not this one—just a list, so I assume they’re all, in one way or another, subtlety working their wiles on my delicate torso—including my hips. Silly? Yes, but there are lots of things in our brave new hi-tech world that are both irritating and worrying. WARNING! Minor rant forthcoming. To avoid, skip to page 753 of this essay.
In my experiments with Vitamin C, I have mixed it with a wide variety of other substances some of which have produced especially pleasing results. One day, on a whim, I mixed a drop of toothache medication with a drop of concentrated Vitamin C solution and liked what I got.

A few weeks later I was doing some shopping at one of the Methyl Hydroxypropyl Super Steroid Mega Shopping Center stores and found, in the pharmacy section, a small bottle of Mouth Pain Liquid. This one has a label that lists and distinguishes active and inactive ingredients. I’m just going to list them, without comment, and let you make up your own silly or indignant comments. First, the active ingredients of which there are only two and they seem quite appropriate for the described purpose: 1) Benzocaine 20% and 2) Compound Benzoin tincture. So far, so good. Now we come to the list of inactive ingredients and I shall number them, even though they aren’t numbered on the label, just for reference, so that I can make a few rude comments at the end of the list, in spite of my promise not to do so.
Inactive ingredients:
1) benzyl alcohol 2) cetyl pyridinium chloride 3) dimethyl isosorbide 4) ethyl cellulose flavor 5) octyl acrylamide acrylates 6) butylaminoethyl methacrylate copolymer 7) oleth-10 |
8) PEG-6 9) propylene glycol 10) ricinus communis (castor) seed oil 11) saccharin 12) SD alcohol 38B (29.6%) 13) tannic acid |
Now remember, this is a liquid, not a pill, so what is all this inactive stuff doing? A little flavoring, I can understand (item #4), but I think I’d rather have a little clove oil than ethyl cellulose. As for item #10, I’d just as soon not have anything at all to do with a plant from which ricin can be extracted. Remember the Bulgarian spy incident when the agent injected ricin toxin into his enemy counterpart using a special tip fitted on the end of an umbrella? The man died a few days later.
There are two kinds of alcohol here (items #1 and #12). Why not just put in a good slug of whisky that will do something helpful? The tannic acid (item #13) is, I imagine, for the compulsive tea drinker and the rest of the stuff sounds like ingredients in some kind of epoxy superglue. We have come to live in a world where the products we use and ingest are filled with substances which are unfamiliar and whose function is mysterious to the non-specialist, especially when we are told that a large number of the ingredients are “inactive.” So, end of rant.
However, I do advise keeping careful notes regarding both active and inactive ingredients in any compounds you are using for crystal experiments. If, at some later time, you use a similar compound which ends up lacking some of the “inactive” ingredients of the compound you previously used or if it has additional ingredients, then you might get some very different results.
Back to Vitamin C. To minimize the difficulties of the additional ingredients used to make the commercial tablets, try to obtain some ascorbic acid crystals. Biological supply houses as well as chemical and general scientific supply firms have them, but often have policies that restrict sales to companies and institutions and will not sell chemicals to individuals. However, ascorbic acid crystals are innocuous enough that if you can’t find a supply company which will sell some to you, you may well be able to convince your local pharmacist to order some for you. Or if your town has a shop that sells supplies for brewing wine and beers, there is a very good chance that it will stock small bottles of ascorbic acid crystals.
There is a variety of methods for preparing slides and the simplest ones seem to work splendidly for ascorbic acid. Dissolve the crystals in distilled water. You can experiment with the concentration, but once you get a moderately concentrated solution, the results don’t seem to vary greatly. When preparing crystals, try always to use very clean slides. “Pre-cleaned” slides are often not so very clean. You can take a dry slide and breathe on it, producing a thin “fog” of distilled water and then wipe it thoroughly with a lint-free cloth or tissue. Another approach is to store some slides in a jar of 70% isopropanol (rubbing alcohol), remove one, and wipe carefully. Place a drop of the ascorbic acid solution on the center of the slide. If it just sits there as a well-behaved drop, then the chances are high that when it dries, you will have a dense, essentially undifferentiated and uninteresting crystalline lump. What I want is a slide where the solution is dispersed in thin layers, streaks, very small droplets in strange configurations and most of these disconnected from one anotherin other words, a very messy slide. The method which I found works best for me is to add a drop of 70% isopropanol and then, with a flat toothpick, quickly mix, stir, and spread the solution across the slide. At first, you may be inclined to think that the turbulence generated by the interaction of the alcohol with the aqueous solution is sufficient to produce a satisfactory dispersal. However, I have gotten more pleasing results with the addition of the toothpick mixing. After experimenting with ascorbic acid both by itself and mixed with other substances, I have come to the conclusion that ascorbic acid is remarkably strange stuff. Since I retired, I find myself regarding almost all my objects of scientific interest in a somewhat different way. Many substances and organisms seem to be more mysterious, complex, and intriguing that ever beforeand, no, I haven’t undergone some mystical conversion. Having more time to think about what I am observing allows me to explore in greater depth and to notice details and interconnections with aspects of other specimens that I had previously overlooked.
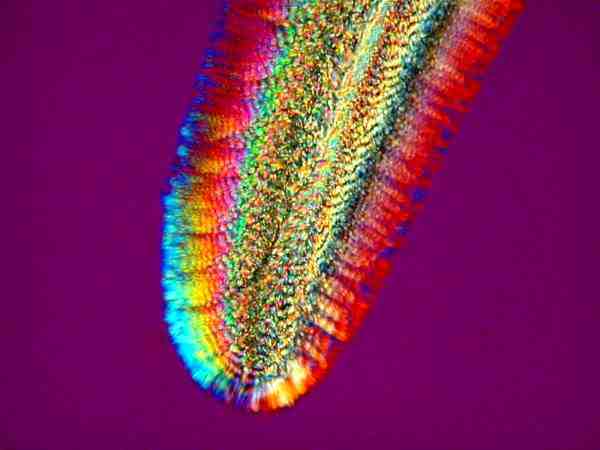
Some of the joys of ascorbic acid are that it produces a range of color from delicate pastels to vivid colors of striking intensity. Sometimes it will produce remarkably delicate clusters of small blade-like crystals

and, at other times, extended mats with “woven” stripes in a series of one color next to another.
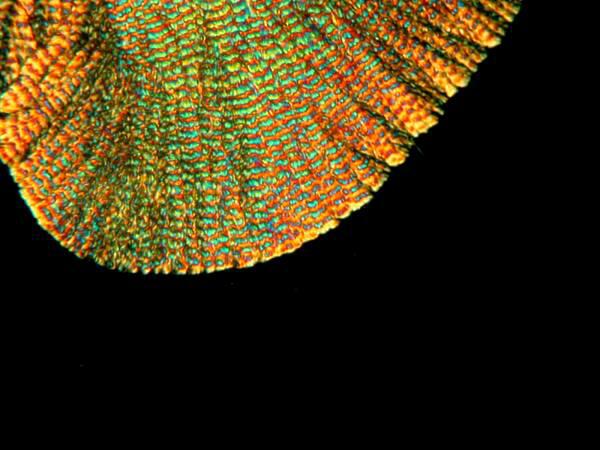
Another fairly typical sort of form produces an “eye” pattern not all that unlike what one finds in peacock tail feathers.
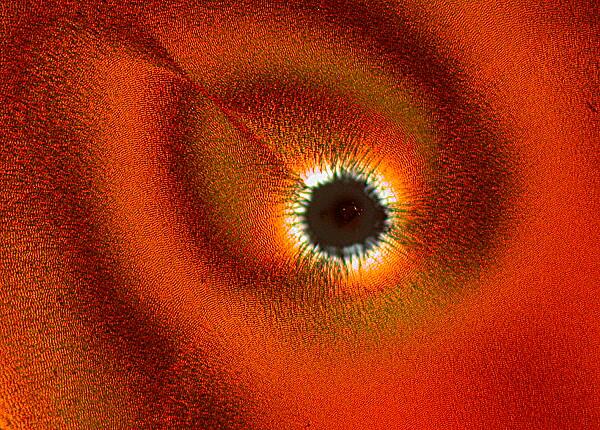
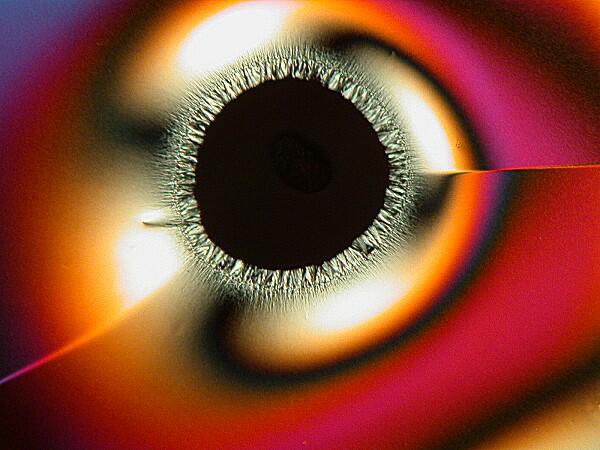
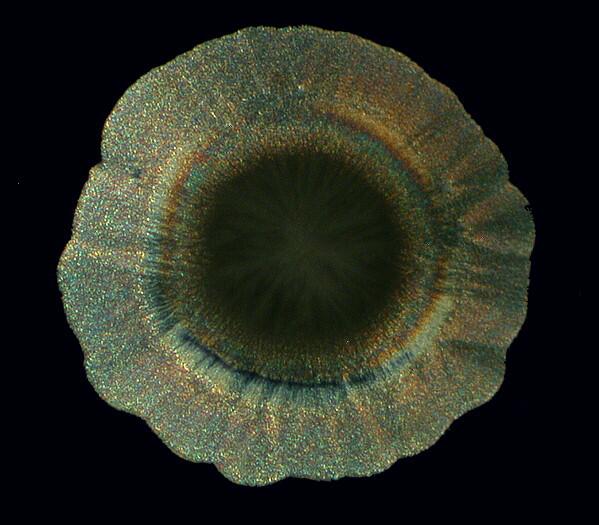
Other times, one will find an “eye” with a black center enclosed by an elongated black oval ring.
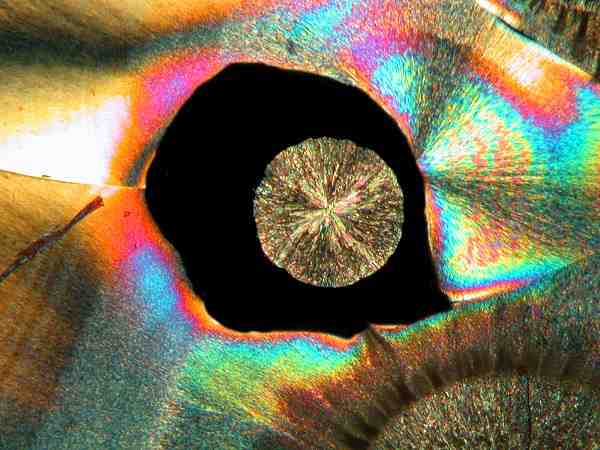
Yet another frequent pattern is the fringed circle or “coin” pattern. Some examples are colored in a fashion so as to appear quite like the marcasite “dollars” that mineral collectors prize.
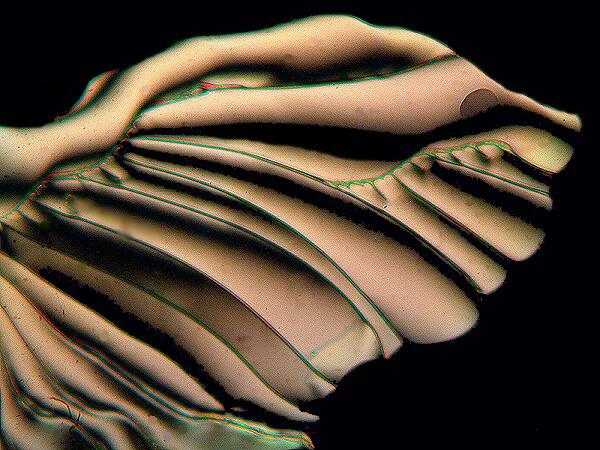
Occasionally you will find an example of one which has partially doubled and looks quite like certain desmids when they are in the process of division. A quite remarkable form came from a slide done as a melt and is alate or wing-like.
After examining several ascorbic acid slides, you will begin to form a mental catalog of certain distinctive patterns and characteristics which will be very useful when you start mixing ascorbic acid with solutions of other chemicals. You will begin to recognize anomalies and certain eccentric patterns which emerge with certain mixtures. For example when I mix ascorbic acid with Janus Green B, I typically get small “black holes” which are disruptions of the patterns formed by ascorbic acid alone.
Another interesting result comes from mixing ascorbic acid with certain common biological stains. such as, Orange G, Methylene Blue,

Acridine Orange,

Alizarin Red S, Malachite Green and a mixture of Orange G and Gentian Violet.

Yet another substance with odd and intriguing properties is the old-fashioned “egg preservative”, sodium silicate. [CAUTION: Sodium silicate is a strong alkaline irritant and can produce severe burns on the skin. It also has a number of incompatibilities and you should inform yourself about those before mixing sodium silicate with other chemicals.] With acids, it forms a gel and thus with ascorbic acid you get the formation of a thin layered gel which crackles on hardening. It needs to be diluted and finding a suitable concentration is, unless you are methodologically compulsive, largely a matter of trial and error, since this chemical reacts quite differently when mixed with various substances. When you get a good mix of ascorbic acid and sodium silicate, you can get some very nice results.
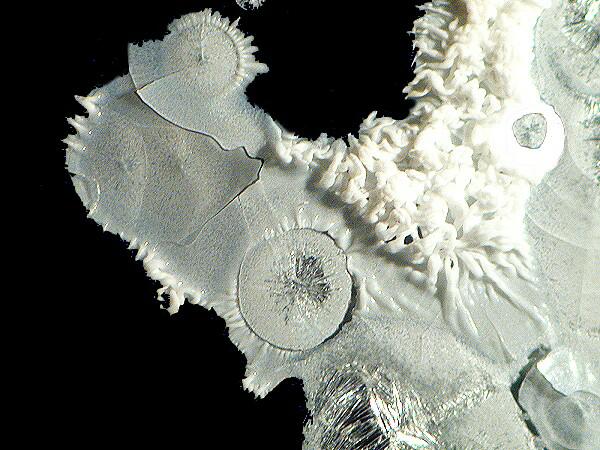
In Part 2, I will look at the results which can be obtained by mixing ascorbic acid with a variety of other substances.
All comments to the author Richard Howey are welcomed.
Microscopy
UK Front Page
Micscape
Magazine
Article
Library
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK