The
inner epidermis of the onion bulb cataphylls
(the
onion skin)
Easy
and not so easy methods to work with
This is an exercise on developing an
understanding of the principles and basic operations in histology,
using the
onion skin as a substrate. I repeat the
usual warning: we treat here with seemingly similar pictures what must
be carefully
examined to appreciate their similitude or difference due to the
different
treatments to which they are subject.
Acetobacter aceti, the responsible
bacteria, is found world wide.
(You
can review in your browser the most famous formulae as the Bouin,
Bouin-Hollande, Duboscq-Brazil, Zenker, Schaudinn, SUSA.)
Injurious liquids which should never
be used in cytological
fixation are acetic acid, chloroform and
alcohol. Acetic acid is nearly the most destructive.... and its use,
except
where chromosomes are being studied, is rarely indicated; any worker
who uses
acetic acid in his fixing mixtures cannot hope to get a correct picture
of any
part of his cell, possibly excepting
the chromosomes (not the
resting nucleus).(pag. 24). Bold
additions are mine.
The references
I have, suggests that acetic is a fast fixative. So I would start with
3
minutes.
Of course I used the same treatment
series I used for the citric, changing the fixative. But
I add
another washing step. Acetic is strong and for the moment I
don’t want
to change the pH of the dye.

And... I cut a
new onion bulb every time I start a test. Old cataphylls could behave
erratically. My wife is creating new dishes, to use so many onions!
Acetic acid 10%
I dilute 2 ml of pure acetic and complete to 20 ml.
Use a hypodermic syringe to do that.
I apply the
usual protocol:
Fixing 3 minutes; first
washing, 4 min; second washing 4
min; staining, 1:30 min; (Ouch! It's
hard to time this) washing with
plenty of water, rinse, mounting in
water.
I stain with my usual solution of Blue 1: 10ml distilled
water, 0.6 ml blue 1
(By the way, the
blue solution must also be fresh (not more than 3 days old) because,
once
diluted the included preservative, the solution is a good culture
medium for
bacteria and yeast)
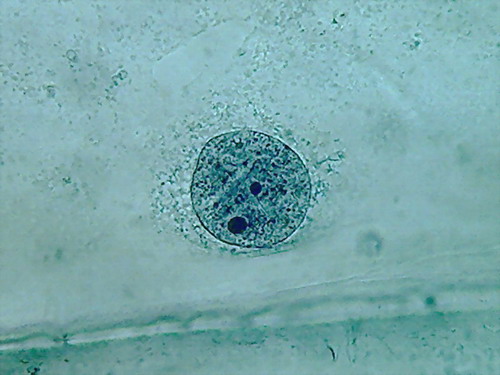
Fig 1 - Acetic acid 10% – 4x objective
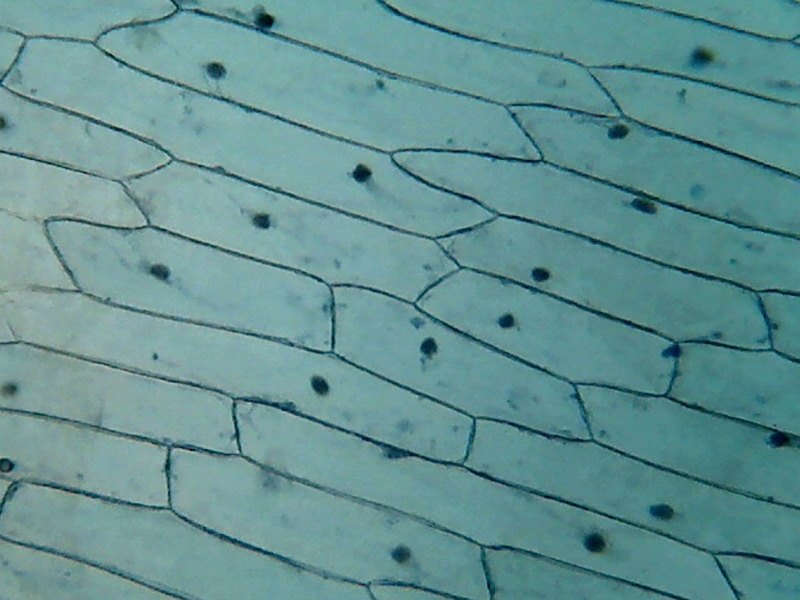
Fig. 2 - Acetic acid 10% – 10x objective
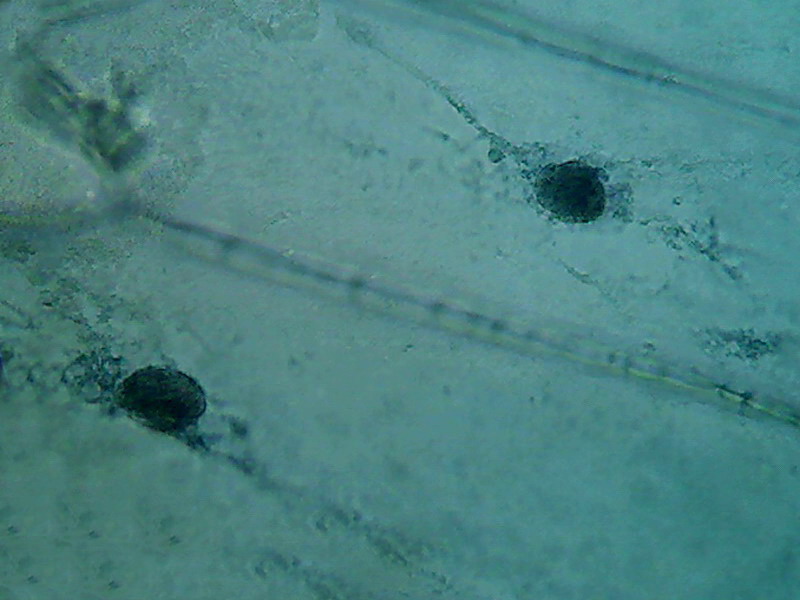
Fig 3 - Acetic acid 10% – 40x objective
|
|
|
|
|
|
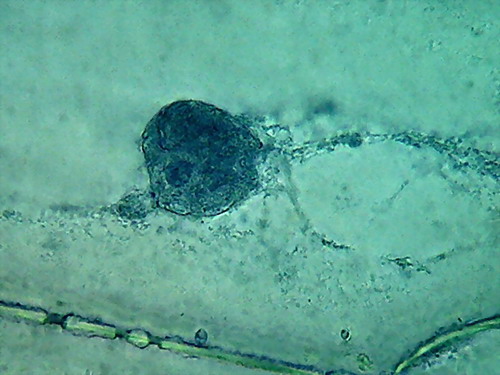
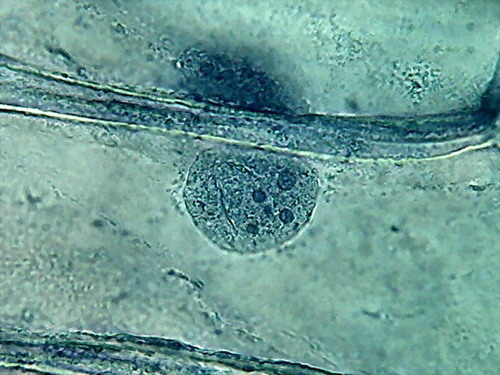
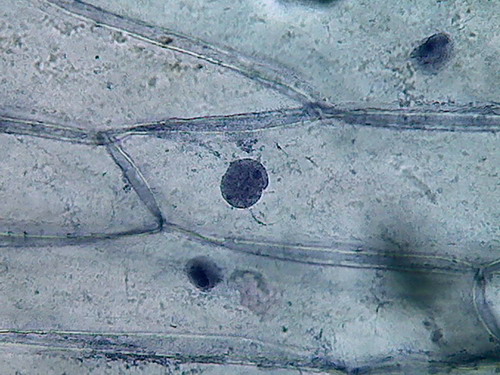
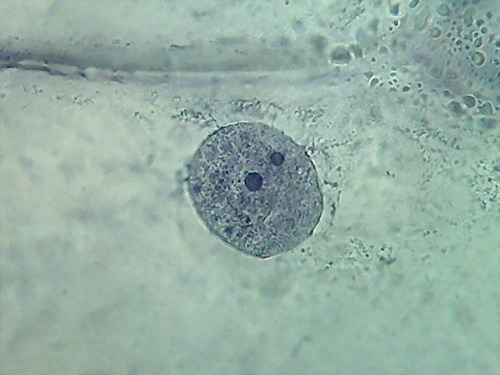
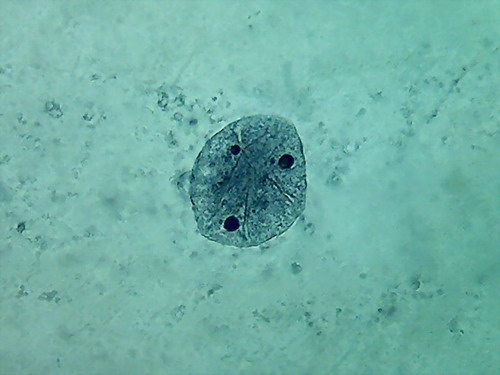
Fig. 4 – Four nuclei - Acetic acid 10% -
100xOI objective – pictures reduced to ¼ of the 1000x image
The second was the hue of the blue, more brilliant and
pure than in any of the essays I did before, even if it is not evident
in the
pictures.
The third was a hard fixed cytoplasm, with visible but
incomplete trails, born on the nucleus and pointing away from it. At
the
parietal layer some holes and ruptures were noted. The parietal layer
is very
conspicuous.
The fourth and the more disappointing were the distorted
nuclei, folded many times on themselves, and with nucleoli difficult to
discern. There is nothing that shows the disc shape I am
accustomed,
even with their typical superficial grooves. I add the evidential
images, as
always, for you to see “almost first hand” the result of the experiment.
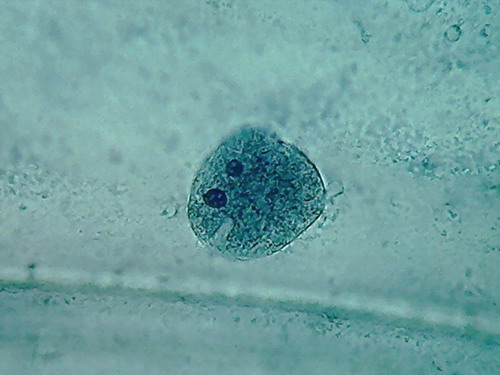
I made more slides to confirm this nasty behaviour. I
add two more pictures to confirm it. They are the best I could pick.
|
|
|
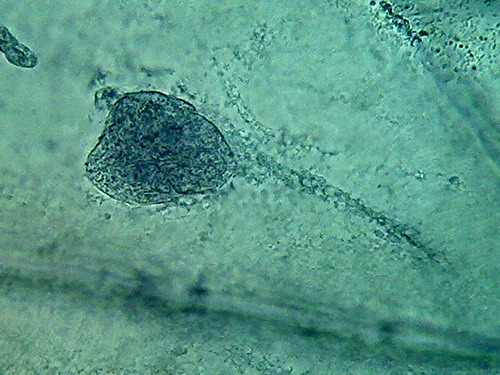
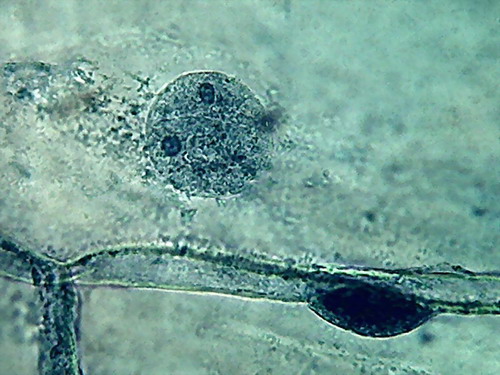
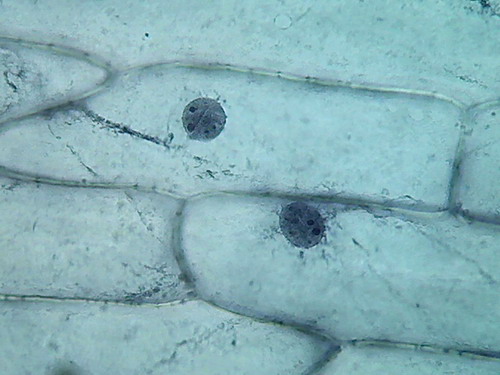
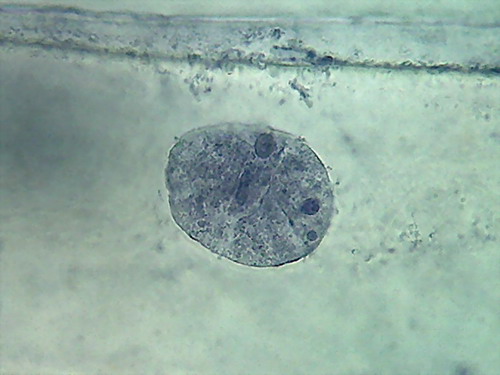
Fig. 5 – 2 more nuclei, 10% acetic,
nucleoli and superficial grooves indistinct
There is not a big difference, the colour is the same,
and the type of fixation of the cytoplasm appears to be equal. The
morphology
of the nucleus is also similar, but in these latest images it seems
more
"classical".
The most important thing to notice is the difference
in appearance of the nucleoli, fixed with this high concentration of
acetic. In
addition being difficult to differentiate, because they have almost the
same
colour and intensity of the chromatin, the form of them is that of an
oval
organelle (instead of circular), without the clear and characteristic
central point
(which is due to a core of central dense fibres, according to the
Transmission Electronic
Microscope), which we have found so far. People say that acetic
dissolves RNA,
and nucleoli are pretty dense masses of filaments of RNA.
In my experience 10% is not a good
concentration for the acetic to be used alone on the onion epidermis.
Fixing with white vinegar
The more easily
found fixative in the domestic dominion, after 60ºC temperature,
is straight
white vinegar.
It is present without restriction in
every kitchen, probably in every
country.
So I must give it a tray. My
commercial white vinegar is an aqueous solution, with 5% pure
acetic acid which has a pH very near
to 2.4. That is just the proportion which
histologists most commonly use, when using acetic acid in their
fixatives, as
you could see in the above quoted formulae!
If we believe
in honesty of the manufacturer, the vinegar
I use is a 5% acetic acid solution, with
0.02% sodium metabisulfite added (to prevent moulds, yeasts, and
nematodes
to develop, which, growing in my mother’s kitchen, in the “mother”** of
pure
wine vinegar, many, many years ago, were so interesting subjects for my
first little microscope).
**For the so,
so young, that has never seen the “mother of vinegar” I translate: It
was a
dense gelatinous mass built by the development of Acetobacter
aceti, which concentrates at the base of the vinegar
bottle. It was many times colonized by Turbatrix
aceti (In those times also known as
Anguillula aceti), the “vinegar eel”, that eats the Acetobacter.
Parts
of
this
mass were transferred by the Cook to new
portions of fresh wine, as a seed to enhance the acetification.
At the
following address you can find a beautiful picture of this little (2
mm) worm
http://4.bp.blogspot.com/-Ddg7WDrYv58/TZ-8Ou3D9HI/AAAAAAAAATQ/y5vYYxQMvYc/s1600/Turbatrix_aceti.jpg
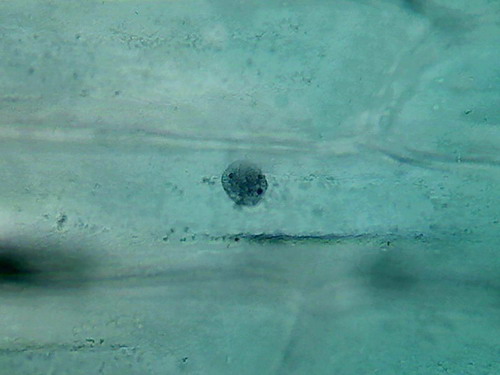
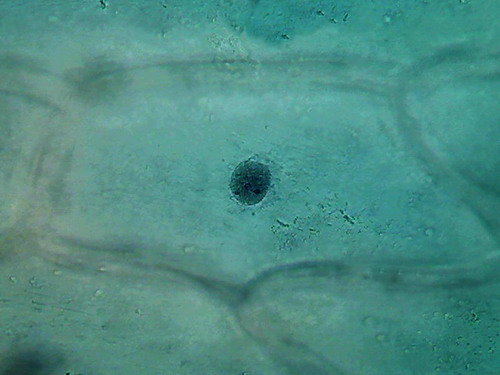
Acetic 5% (vinegar)
I opened a new
bottle of commercial white Vinegar, and started with the straight
concentration, fixing a fresh cut cataphyll
fixing 3 minutes, first
washing 4 min, second washing 4
min, staining 1:30 min, washing, rinse, mounting in
water.
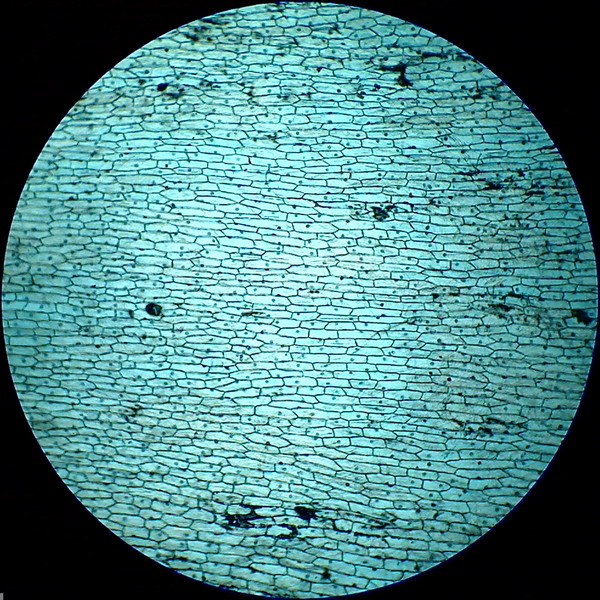
Fig. 6 - 4x obj. shows some bubbles
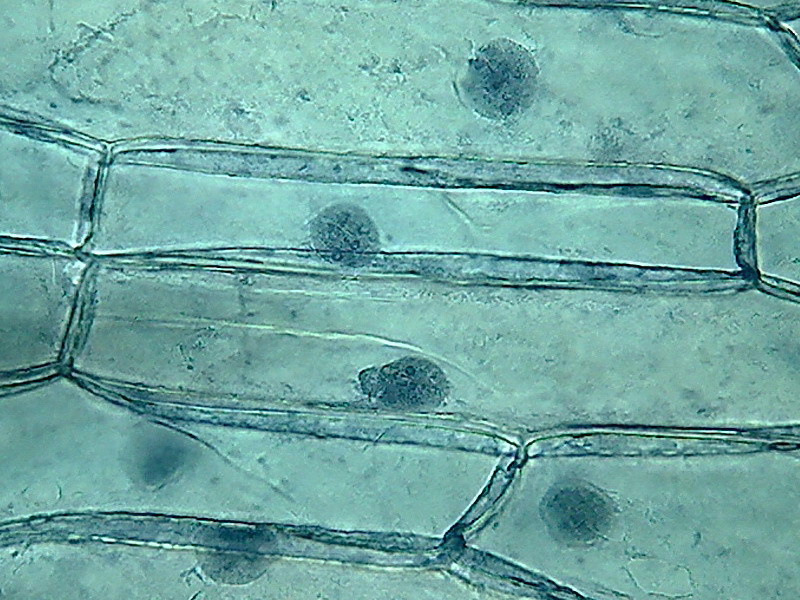
fig. 7 40x obj.
|
|
|
|
|
|
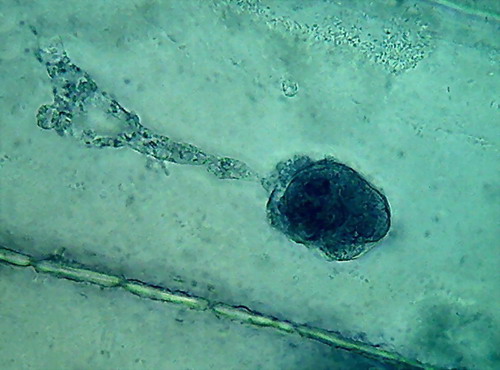
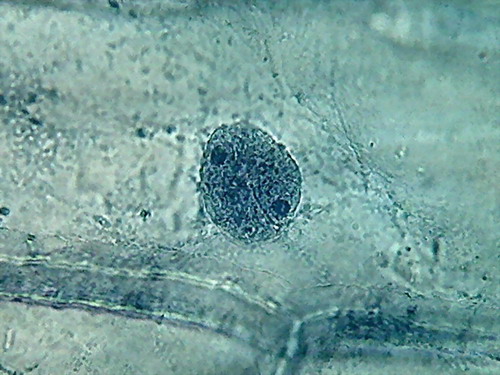
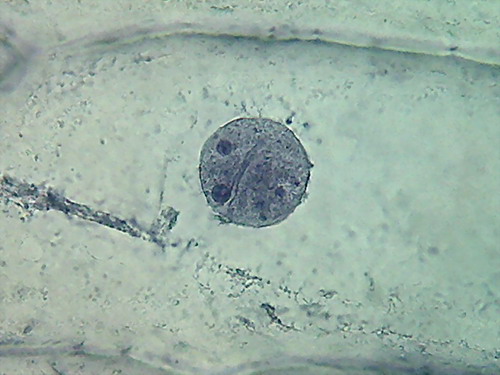
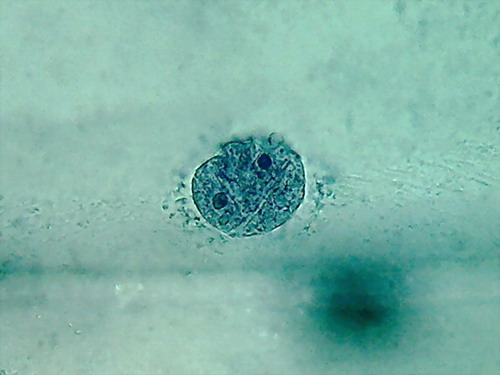
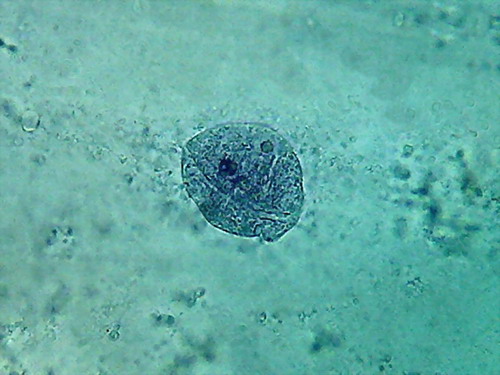
Fig 8- 5% acetic – 100xOI obj. -
Images reduced to
quarter of the 1000x pictures. The colour intensity of the nuclei is
not bad.
The nucleoli are better fixed than with citric acid, and many have the
typical
aspect, with the clear central point. But the cytoplasm is set in a
very dense,
unnatural form, as "crumpled". I can’t see well formed individualized
granulations (mitochondria, leucosomes, sphaerosomes).
2%
acetic acid
Fixing: 3 min, first washing
4’, second washing 4’, staining 3 min, washing and rinse.
Thoroughly washing of fixative, and of dye.
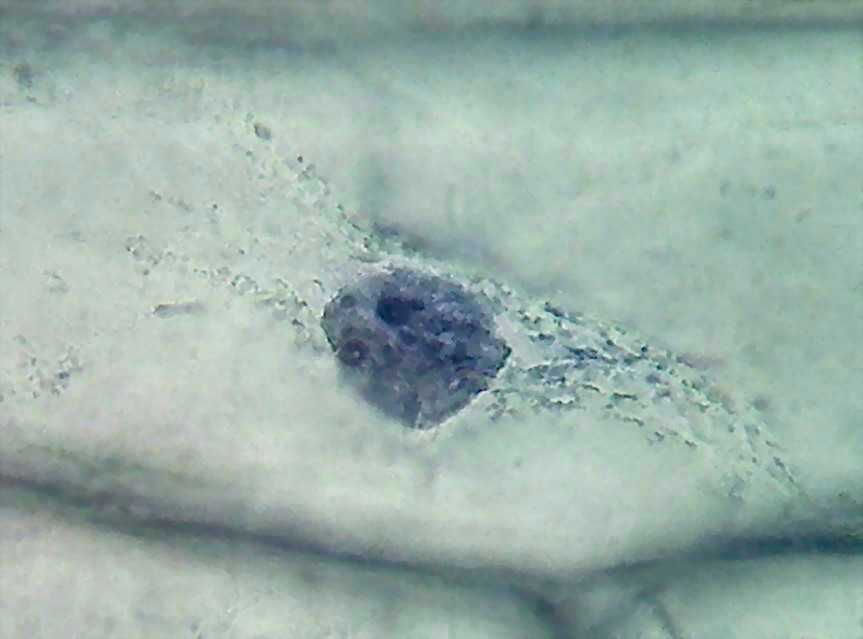
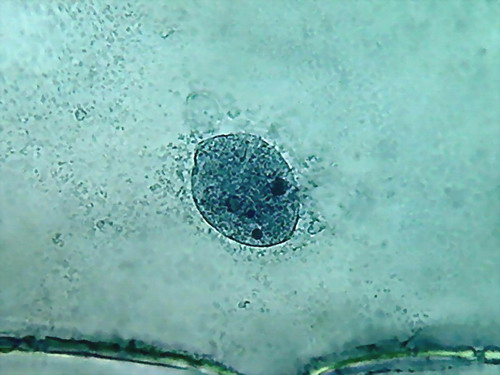
. 9 - 100x obj – A rupture in the cytoplasm over the nucleus. CbZP
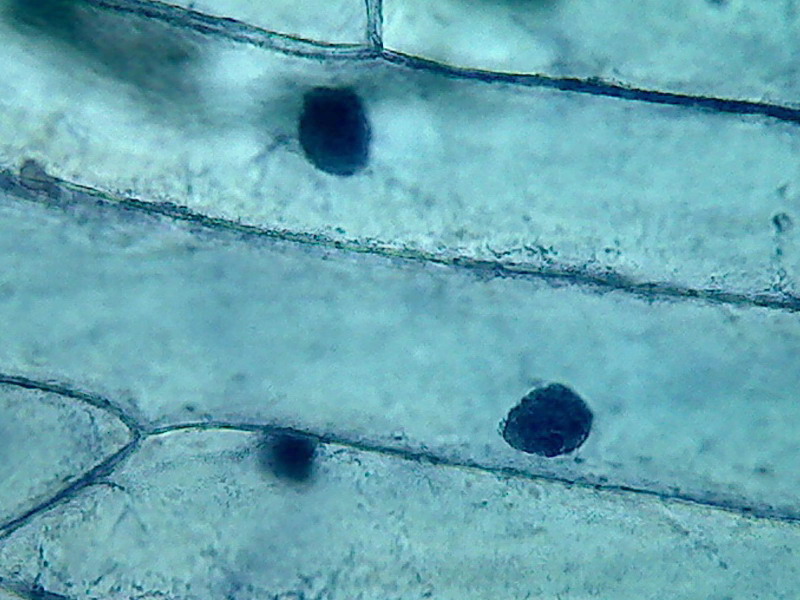
Fig 10 – 2% Acetic - 40x – very dark
Regressive staining - When the time of staining is short, (is
hard to stop staining exactly at 1:30 min) it is difficult to manage
accurately
the depth of the colour. So, some
histologists (as I did here) prefer to stain deeply and afterwards to
remove
part of the stain with a reagent that dissolves the dye slowly,
controlling one
or more times the discoloration. This is the basis of what is known as
a regressive colouration.
On the other hand the colouration protocol we used so
far is known as progressive.
With histological tissue sections, which are fixed to
the slide, is simple to pass the preparation to the decolourizer, take
it out
periodically, verify the intensity, return it to the destaining
solution, and
so on. When the effect is the one the histologist prefers, he stops the
action
washing the removing agent, and proceeds with the rest of the protocol.
The onion skin is more difficult to manipulate. After
being fixed in acetic (and coloured, and cut out from the handles to be
mounted)
it is somewhat rigid. The coverslip of the wet mount can be removed,
with the
aid of a mounted needle, and the piece of epidermis can be slipped with
care to
one little shallow dish with the destaining fluid. To recover it, slip the coverslip under the epidermis with one hand, and slide the epidermis over it with a mounted needle.
Believe me; the manoeuvre is easier than its
description.
The removing fluid I used is a weak alcohol solution
at 30%. It could take 30 to 40 seconds to obtain a good colour density.
|
|
|
Fig. 11 – 2% - 40x – excess stain
removed with 30%
alcohol
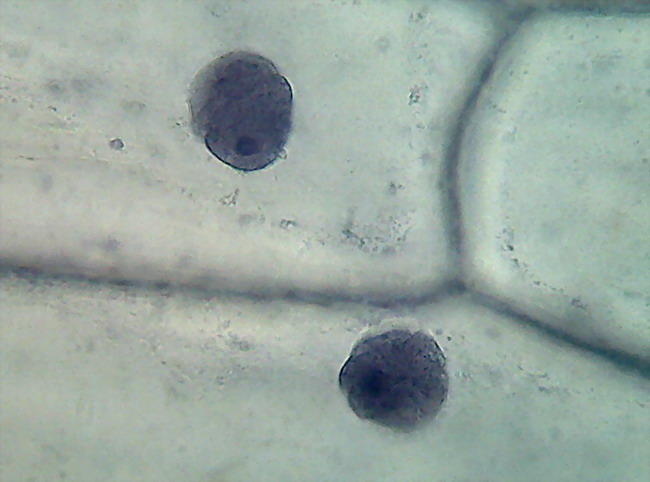
Fig. 12 – some, but few, nuclei had
plied shapes, with
a diffuse structure
|
|
|
|
|
|
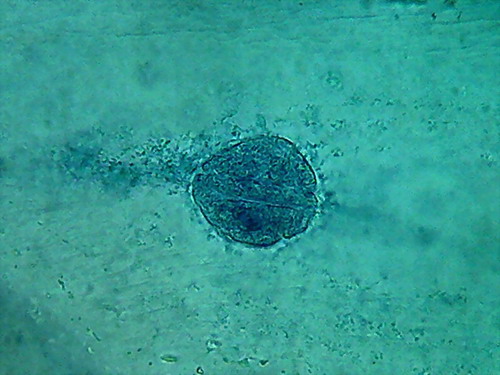
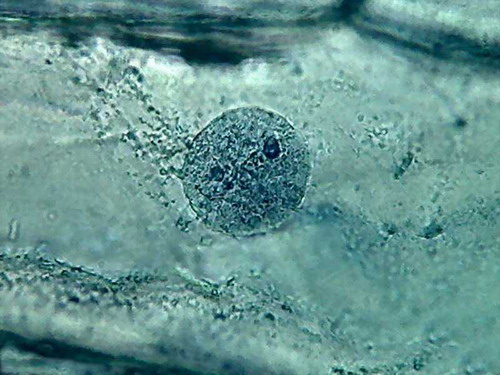
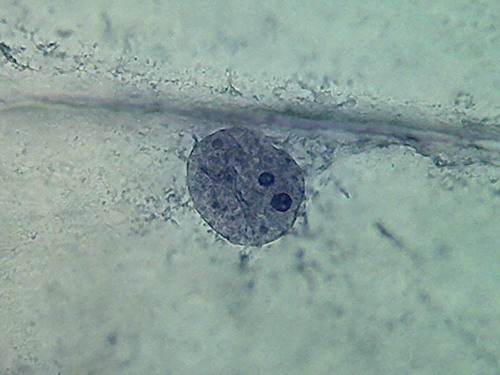
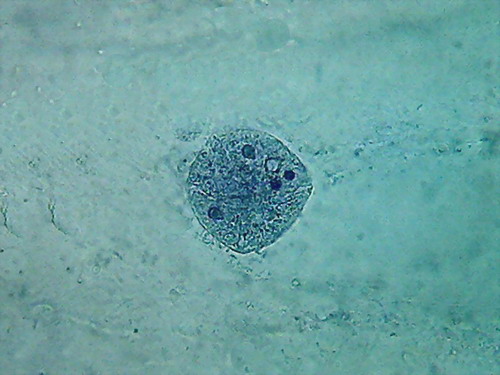
Fig 13 – 2% acetic acid - ¼
reduced images of 4 nuclei.
100x obj., after decolouration.
The cytosol is a bluish gray mass
with folds, in which mitochondria (RNA)
and other granules are absent, and which often show “holes” in a
totally
abnormal fashion. The clear streaks of cytosol distinguished some times
with
iodine, and even 60ºC water, are absent.
The comparison between 5% and 2%
shows however advantages for the latter,
at both the nuclei and cytoplasm levels.
So, before leaving the acetic acid
used alone, I will try a 1 % solution
as my last exercise.
Fixing: 5-6 min, first
washing 4’, second washing 4’, staining 2
min, washing and rinse. Verify
colour. Alcohol 30% (if needed), rinse. Wet-mount
With similar characteristics to the
2%. Coloured nuclei. Distinct nucleoli.
Dense cytoplasm, with folds, grooves and breaks which are difficult to
interpret. I don't show the images with 4x and 10x, as they are as
shown before.
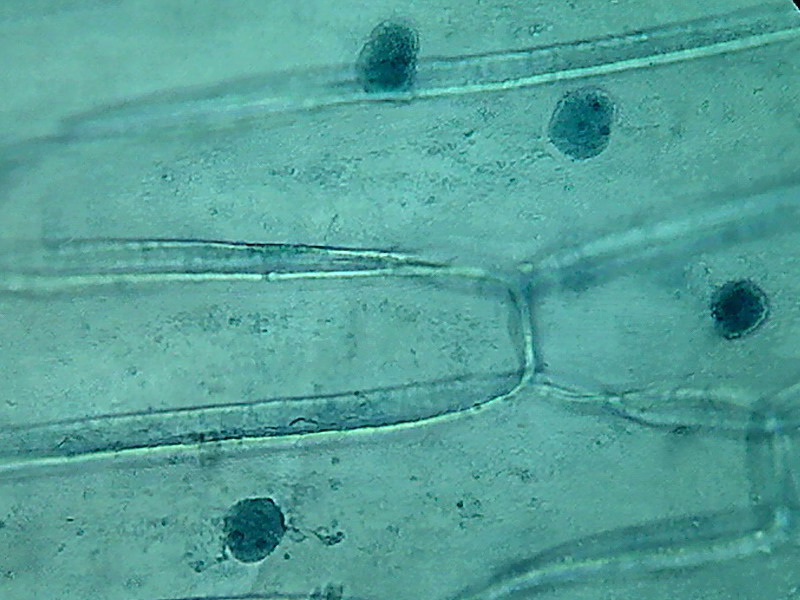
Fig 14 – 40x, 1% acetic
|
|
|
|
|
|
Fig 15 – 100x – reduced to ¼ -
1% acetic
Although the cytoplasm looks
something more normal (if I know what a
normal cytoplasm is? I must say instead: more like with the citric, or
even
with iodine) it still shows artifacts* that are disturbing. The worst
are the
cracks and holes, evidently of the parietal layer of the cytosol. The
nuclei do
not have a bad morphology, and the nucleoli continue to be well
featured.
* artifacts are morphological details
that do not seem
natural, and are caused by a improper action of the reagent, or even by
particles foreign to the preparation, included in it accidentally.
Acetic 1% from pure
acid
To test the confidence I can put on
the vinegar I test also a 1% dilution of the pure
acetic acid.
Fixative, 3’; washing1, 4’; washing2, 4’; staining, 2’; washing; rinsing
|
|
|
16 – 40x –acetic acid, pure, 1%,
aqueous
|
|
|
|
|
|
17 – Nuclei with 100x obj., 1% pure acetic,
aqueous, pictures reduced to 1/4 of size.
2% and 1% are clearly superior to the higher
concentrations. The best images of nuclei I have are from the 2%
acetic, after
regressive colouration. More than 100 years ago Flemming (an enthusiast
of the still
now highly regarded strong osmic and chromic acids as cytological
fixatives)
selected for fixing nuclei in animal
tissues, acetic in concentrations as low as 0.2% to 1%.
And the cytoplasm? As for citric acid, acetic is a nuclear
fixative. Until now, only the Iodine tincture gives a somewhat more
detailed
representation, poor as it is, of the cytoplasm, more crisp and clear
than the
mere row of granules the other described fixatives showed (see the
April
article,
fig.15 and 18).
The "ceviche’s way" has
exhausted the possibilities. Both citric acid and acetic have shown
their
ability to fix the onion epidermis cells showing its basic elements:
cell wall,
cytoplasm (usually visible as a more refractive parietal layer attached
to the
cell wall), nucleus with its disc-shaped structure, and their 2 to 4
nucleoli
disk-shaped, or spherical, with a clear point in its central area (of
course,
when they are well fixed and focused). Neither of them (citric or
acetic) showed
the cytoplasm with the same detail that the tincture of iodine does.
About the pejorative statement of Bolles
Lee, history has only partially confirmed it, because as I have told
you,
acetic acid, alcohol, and chloroform, are in the formulae of very well
known “old
fixatives” used until now. And even if it is not the best in the world,
the
images I have taken from the epidermis fixed even in diluted vinegar,
are
good
representations of the onion cell nucleus.
Acetic
(and the other reagents) should have been tamed in some way.
I have a consolation. The games I’m
playing now for my own illustration,
were surely tested by the first microscopists, but their
work was
really frontier scientific research. They were building, and sharing,
the first
bricks of modern histology. Testing, accepting, and discarding results,
some
times being right, some times being wrong, step by step, they
constructed (more
than a century ago) the solid foundations of the extraordinarily useful
techniques and science of modern histology.
Science, says someone, “is an error
that is every
time a lesser error”
For
now, I must say goodbye to the ceviche!
But...
there is a very big problem, you know...
Most of the professional fixatives of
two decades ago (those which I
have listed before in the introduction to this article) are now banned.. They have very toxic (mercury
dichloride), or carcinogenic (formaldehyde), or addictive (chloral hydrate) components, or ingredients which
may be used... to
make terrifying explosives! (picric acid and picrates). Those
components are
not being sold to amateurs, and in many cases not even to professionals.
But, trust me; the holy fathers of
histology had devised, in the very
old times of the last half of the 19 century, some premonitory answers,
which
can now be applied to this modern social
behaviour, then totally unexpected. Let me start exploring them.
And it's very disgusting, but air
bubbles have returned!