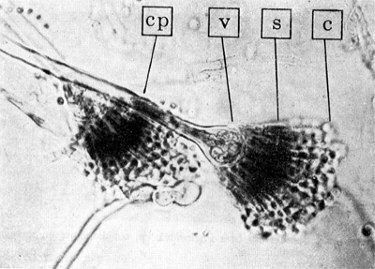
Key to Aspergillus shown right
and below.
|
 |
Moulds
by Mike Morgan, UK
The term "mould" is a common name having no taxonomic significance. It is applied to a variety of fungi which grow as semi-microscopic organisms, and whose mycelium tends to form a loose meshwork rather than a dense tissue. Thus the moulds are distinguished from the large fleshy fungi, the mushrooms etc. Moulds may belong to any of the classes of Fungi, but actually the great majority of species are either Phycomycetes or Fungi Imperfecti (form-class Deuteromycetes).
Moulds will be familiar to everyone as the growths that appear so frequently upon fruits and other foodstuffs. Almost always these moulds are species of Aspergillus and Penicillium. These are moulds, which reproduce by free spores, or conidia, and are distinguished by the characters of their spore-bearing stalks, or conidiophores. In Aspergillus the conidiophore arises form a vegetative mycelium called a foot-cell. This is larger than the filament of mycelium and when the spores are ripe it is often empty, i.e., it contains no protoplasm. The stalk itself is non-septate, without cross-walls. It ends in a swollen portion, the vesicle. From this expanded tip the conidia are formed in chains from the ends of a large number of little stalks, the sterigmata. In some cases the sterigmata are branched, the spores arising from the branches, or secondary sterigmata.
Key to Aspergillus shown right
and below.
|
 |
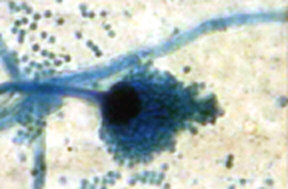 Aspergillus
species are widespread, occurring in soil and also upon vegetable
matter. The species grows best at rather high temperatures, 35 -
40°C. Aspergillus niger is probably the most common
species. It is widespread and is one of the most troublesome
contaminating moulds in the microbiological laboratory. It forms
very large black globular spore heads. The sterigmata are
branched. The conidia are round, black and prickly on the
surface.
Aspergillus
species are widespread, occurring in soil and also upon vegetable
matter. The species grows best at rather high temperatures, 35 -
40°C. Aspergillus niger is probably the most common
species. It is widespread and is one of the most troublesome
contaminating moulds in the microbiological laboratory. It forms
very large black globular spore heads. The sterigmata are
branched. The conidia are round, black and prickly on the
surface.
Aspergillosis refers to a number of disease states in human beings that are caused by Aspergillus. Aspergillus niger, A. flavus, and A. fumigatus, result in a range of reactions. Aspergillus is prevalent in the air and inhalation is common. Persons with a normal immune system are able to fight any infection. Aspergillosis is almost entirely limited to those with a compromised immune system, caused by drug therapies or disease. Microscopic identification of colonies and the characteristic septate hyphae and spores in the samples of sputum will confirm the diagnosis of the disease.
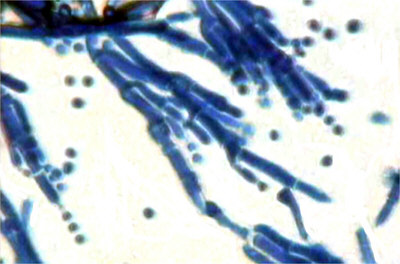 Penicillin
is still one of the most widely used antibiotic agents. It is
obtained from the Penicillium mould (shown right). In
1928 Alexander Fleming noted that the growth of colonies of the
bacterium Staphylococcus aureus was inhibited in those
areas of a culture that had been contaminated by the mould Penicillium
notatum. Fleming isolated the mould and grew it in a fluid
medium. He found that this produced a substance capable of
killing many of the bacteria that cause disease in humans. In
1940, research workers developed an injectable agent for
therapeutic use.
Penicillin
is still one of the most widely used antibiotic agents. It is
obtained from the Penicillium mould (shown right). In
1928 Alexander Fleming noted that the growth of colonies of the
bacterium Staphylococcus aureus was inhibited in those
areas of a culture that had been contaminated by the mould Penicillium
notatum. Fleming isolated the mould and grew it in a fluid
medium. He found that this produced a substance capable of
killing many of the bacteria that cause disease in humans. In
1940, research workers developed an injectable agent for
therapeutic use.
In Penicillium there is no foot-cell, the stalk arising from an undifferentiated cell of the mycelium. The conidiophore is septate and ends in a whorl of short branches, the metulae, each of which bears a whorl of little branches or sterigmata, which forms the conidia. The spore heads of Penicillium are seen to be looser than those of Aspergillus. They are more brush-like in appearance as compared with the compact globular or cylindrical spore heads of Aspergillus.
Species of Penicillium are more common than those of Aspergillus. They have, however, a lower optimum temperature, 25 - 30° C, and are not so troublesome in the microbiological laboratory, which commonly use a 37° C, incubator. Many species of Penicillium form green conidia. P. expansum is one of the most common of the green species of Penicillium causing a rot of apples. P. roqueforti is also a green species forming the mould spots in Roquefort cheese. P. camemberti forms a woolly, white aerial mycelium, with conidia which are of a light grey-green colour. On lemons and oranges may be found P. italicum (blue-green) and P. digitatum (olive-green).
Microscopic Preparation of the Moulds
Moulds are best stained with lactophenol cotton blue for examination. A thin line of the stain is placed on a microscope slide. A piece of sellotape is then applied to the mould growing on the fruit etc., and this is then carefully placed on the stain-containing slide, and examined under the microscope. In the absence of lactophenol cotton blue, a fairly good preparation can be made using blue ink.
References:
The Biology of Bacteria, A. T. Henrici revisd by E. J. Ordal, 3rd Edition 1948 D.C.Heath and Company.
Biology Staining Schedules, R.R. Fowell, 9th Edition 1970 H.K.Lewis & Co. Ltd.
Acknowledgement:
Aspergillus annotated image is reproduced from 'The Biology of Bacteria' with thanks to the publishers Houghton Mifflin.
Comments and feedback to the author Mike Morgan are welcomed.
Published in January 1999 Micscape Magazine.
Please report any Web problems
or offer general comments to the Micscape Editor,
via the contact on current Micscape Index.
Micscape is the on-line monthly
magazine of the Microscopy UK web
site at Microscopy-UK