|
|
A Gallery of Ammonium Sulphate Photomicrographs (NH4)2SO4 (using
a variety of illumination techniques) |
|
|
A Gallery of Ammonium Sulphate Photomicrographs (NH4)2SO4 (using
a variety of illumination techniques) |
Ammonium
sulphate is one of many compounds used to prepare agricultural
fertilizers. It is also used as a component in nutrient mixtures
for bacterial cultures in wastewater treatment. In addition, this
salt is a component of fire-extinguishing powders.
Historically, this compound was one
of the first types of “synthetic nitrogen” fertilizer manufactured and
used in large quantities. Its popularity was due to the fact that
it was a by-product of the town gas works in many countries.
The white crystals have a
moderately high melting temperature – about 235 oC.
This would normally discourage me from attempting to prepare a melt
specimen, but in this case, I persevered. A small quantity of the
solid was placed on a microscope slide, covered with a cover-glass, and
heated gently over an alcohol lamp. As soon as the solid melted,
and had formed a thin liquid film, the slide was removed from the heat
and allowed to cool slowly.
Note:
The MSDS safety document concerning ammonium sulphate states:
Contact with strong oxidizers may cause
fire or explosion.
Harmful if swallowed.
Eye, skin and respiratory
irritant.
Under the conditions described
above, many long, thin rectangular crystals form that have rounded
ends. Elliptically polarized light was used to produce the gray
background in the following image. (Crossed
polars + two lambda/4 plates)

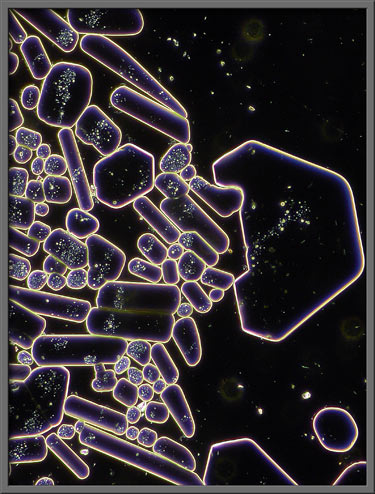
The two images below show
approximately the same area on the slide. The first uses
dark-ground illumination to delineate the edges of the crystals,
whereas the second uses elliptically polarized light. (Crossed polars + two lambda/4 plates)
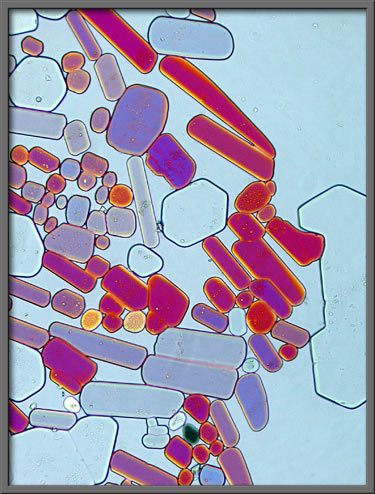
If a phase-contrast condenser is
combined with an ordinary, (non-phase) objective, the image produced
has a distinctly three-dimensional character.
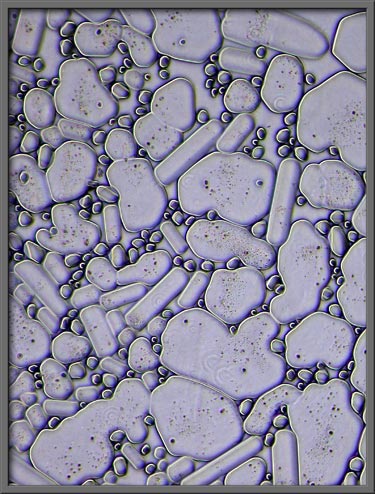
The same type of area, visualized
by the use of a dark-ground condenser, looks like those shown in the
two images below.
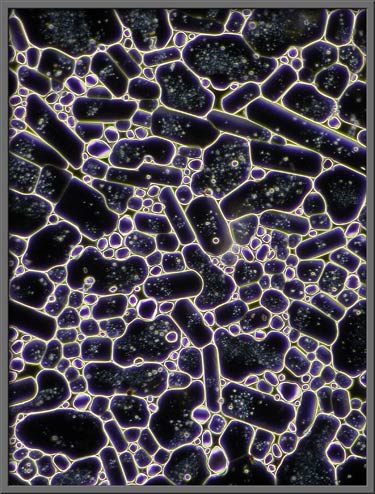
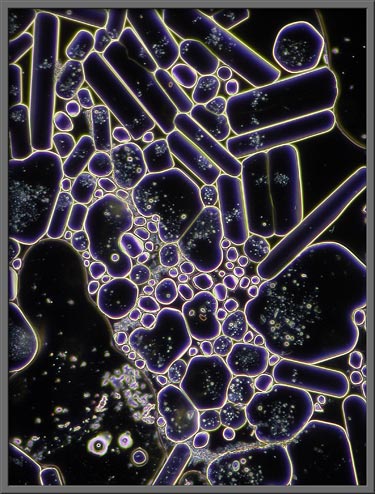
A phase-contrast condenser combined
with a proper phase objective (PHACO) shows interesting structural
details. Note that the three images that follow are of a higher
magnification than the others in the article.

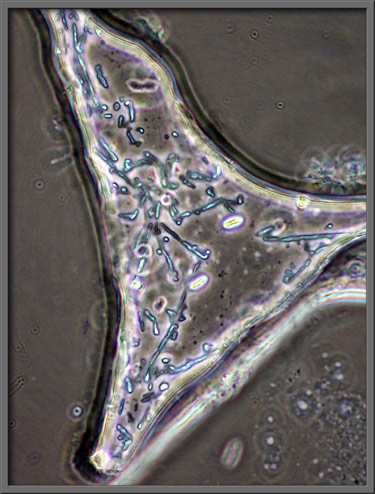
Elliptically polarized light was
used to illuminate the section of slide shown below at low
magnification. (Crossed polars + two
lambda/4 plates)

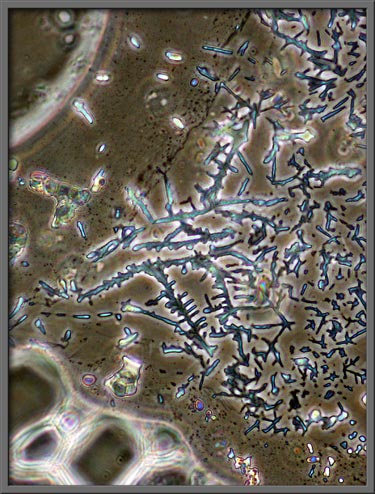
Higher magnification images of a
similar field follow. (Left image: Crossed
polars + two lambda/4 plates) – Right image: (Crossed polars + lambda/4 plate + lambda
plate) Note:
The first image in the article is the same as the first image below,
but it had Photoshop’s “Invert (colour)” command used on it.)


Still higher magnification reveals
the following. (Crossed polars +
lambda/4 plate + lambda plate)
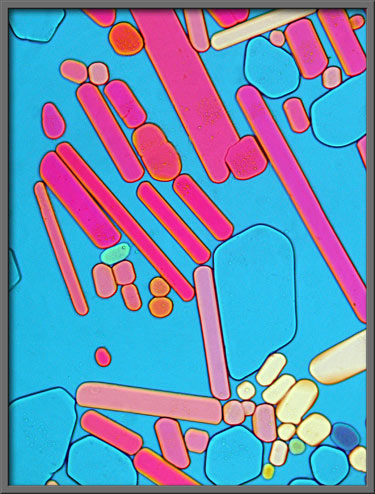
Ordinary
plane-polarized light provides the ultimate contrast between colourful
crystals and background. (Crossed
polars)
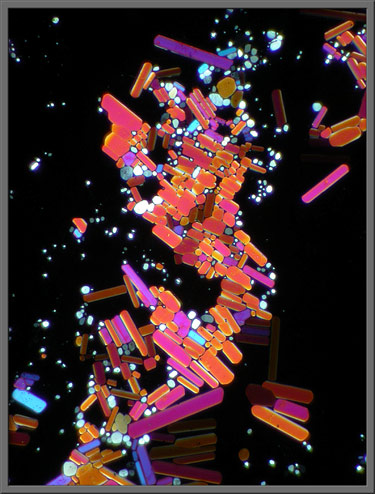




This common industrial chemical’s
high melting temperature makes the preparation of melt specimens
particularly difficult, but I feel that the results are worth the
effort.
Photomicrographic
Equipment
The images in the article were
photographed using a Nikon Coolpix 4500 camera attached to a Leitz
SM-Pol polarizing microscope. Images were produced using several
illumination techniques: dark-ground, phase contrast and polarized
light. Crossed polars were used in all polarized light
images. Compensators, (lambda and lambda/4 plates), were utilized
to alter the appearance in some cases. A 2.5x, 6.3x, 16x or 25x
flat-field objective formed the original image and a 10x Periplan
eyepiece projected the image to the camera lens.
Published in the
January 2008 edition of Micscape.
Please report any Web problems or
offer general comments to the Micscape
Editor.
Micscape is the on-line monthly magazine
of the Microscopy UK web
site at Microscopy-UK
© Onview.net Ltd, Microscopy-UK, and all contributors 1995 onwards. All rights reserved. Main site is at www.microscopy-uk.org.uk with full mirror at www.microscopy-uk.net .