 |
A Gallery of Benzoin Oxime Photomicrographs ( using polarized light and dark-ground illumination ) by Brian Johnston (Canada) |
 |
A Gallery of Benzoin Oxime Photomicrographs ( using polarized light and dark-ground illumination ) by Brian Johnston (Canada) |
Of the several hundred chemical compounds that I have photographed over the past 30 or so years, benzoin oxime stands out as one of the most beautiful when viewed under a polarizing microscope. Unfortunately, the depth of colour in the best slides is poorly portrayed on the printed page or computer screen. Only colour slide film, when projected, gives a true representation of what is seen through the microscope's eyepieces. Nevertheless, I hope to give you a visual "taste" of what can be expected if you choose to investigate this uncommon organic compound.
Before complex machinery was developed for chemical analysis, benzoin oxime was used in the gravimetric (by mass) determination of the element molybdenum. It is usually supplied as a white crystalline solid with a melting temperature of about 155 degrees Celsius. The low melting point makes it easy to produce a melt specimen by placing a few crystals on a slide, and covering with a cover glass. An alcohol lamp is used to heat the slide gently until the melted compound flows to fill the space between slide and cover slip. It is very important not to overheat the sample. The tendency is to hold the slide close to the flame in order to speed up the process. Experience has taught me to hold the slide at least three or four flame lengths above the tip of the flame and to wait patiently until melting begins. At that point the slide is moved still farther away until the crystals have all melted. Unless this technique is followed, voids tend to form in the crystal field that spoil the finished result. Slides prepared in this way can be remelted many times. (All of my benzoin oxime slides were originally prepared twenty-six years ago, and I was able to recycle them through many melting-recrystallization steps to produce the images in this article.)
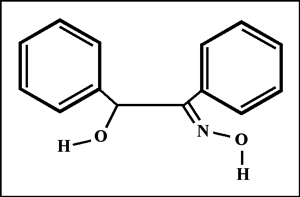
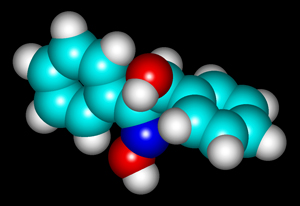
The structural formula and molecular structure are shown below. You may notice that the shape of the molecule is different than that of the structural formula. "HyperChem" software allows the user to perform a "geometry optimization" on the molecule that takes into account the attractions and repulsions between atoms to produce a "likely" orientation. (Other "likely" orientations are possible.)
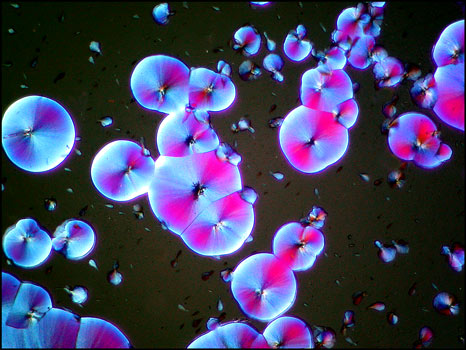
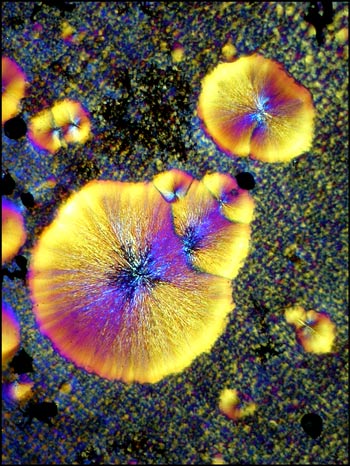
All of the polarized light photomicrographs in the article were taken with the polarizer and analyser adjusted to give a black background (extinction position). In many, compensators were used in the microscope to alter the colour range of the field of view. The two examples below illustrate how lambda/4 or lambda plates can change the appearance of a particular crystal field.


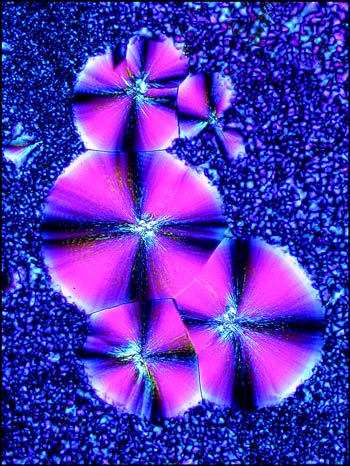
The previous photomicrographs are very typical of fields produced by this compound. The circular formations begin to grow as soon as the slide begins to cool and their diameter increases for a few minutes. For some reason this process usually stops before the circles have completely filled the field. For several hours the slide may look like the example below.
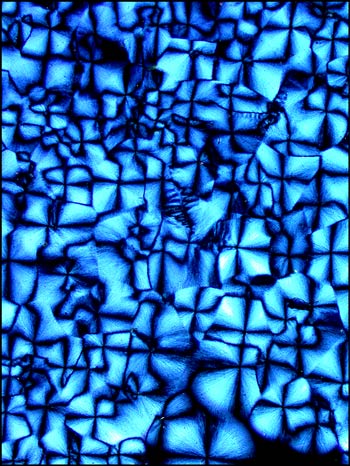
Eventually however, (usually within a day), the material between the circles crystallizes into what appears, at low magnifications, to be a granular pattern. This can be seen in the two images below.
At higher magnifications, this granular appearance is resolved to be many amorphous crystals that grow to fill the space between the circular forms.
Each of the circular forms in the image below on the left, has black lines and spray shaped areas that are quite distinctive in many benzoin oxime photomicrographs. The image on the right was produced using a dark-ground condenser instead of the polarizing condenser utilized in the other examples. It seems that the crystal growth is so thick in this area that it is effectively opaque and appears black in the polarized light image.

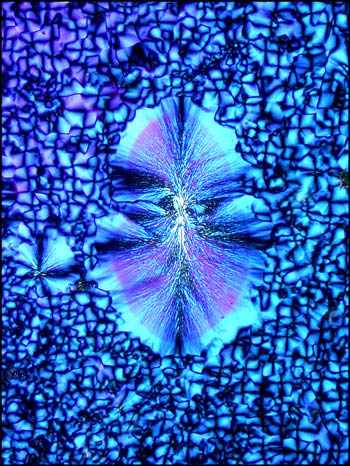
Another image of an area near the edge of a cover glass shows another distinctive pattern.
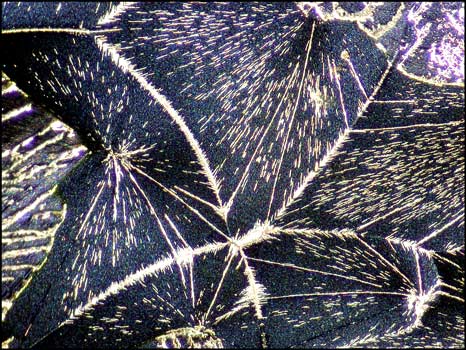
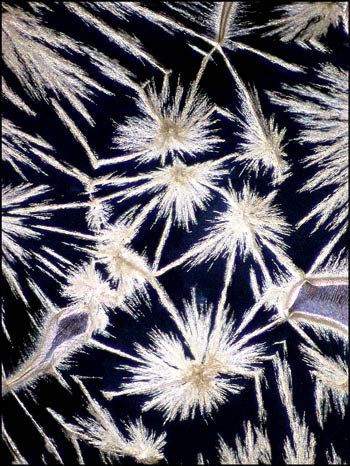
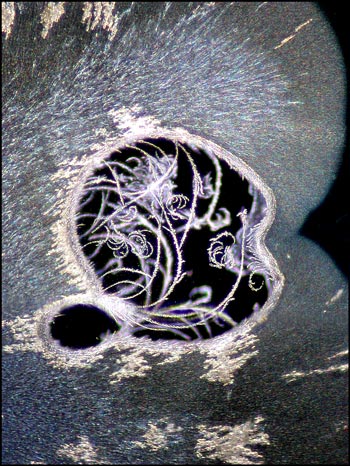
Even seemingly ugly blotches on an image may provide a surprise when studied closely at higher magnification. In the image on the left, in the bottom left quadrant, there is a circular black spot. Other slides display similar imperfections. An image of one of these, using dark-ground illumination, reveals the fascinating view on the right. My guess is that for whatever reason, a void formed between slide and cover slip during crystallization. The heat must have been sufficient to vapourize some of the benzoin oxime which then sublimed (gas directly to solid) forming the curlicues in the image. When viewed in the right light, even a blemish is beautiful!
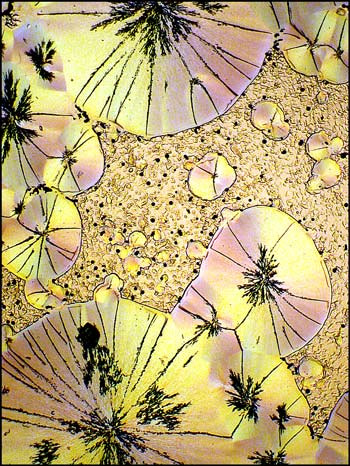
Benzoin oxime has proven to be a rich provider of interesting images. The three photomicrographs below show the diversity of the crystal fields that can form as the melt solidifies.


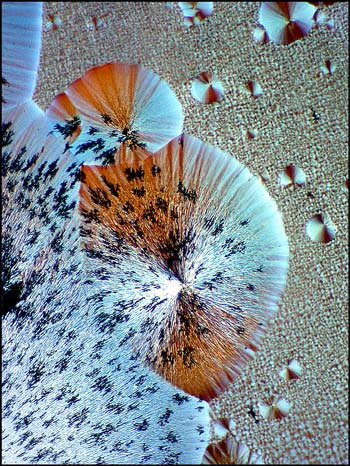
Although the forms shown so far in this article are very characteristic of benzoin oxime, others are sometimes observed. The image below shows a crystal pattern that is not so typical of the compound.

My goal in this series of articles for Micscape, is to show the reader a glimpse of an extraordinary world, normally hidden from view. Benzoin oxime is, I believe, a destination worthy of investigation.
Caution should be taken when working with benzoin oxime. It may be harmful if swallowed or inhaled. The compound may cause irritation to skin, eyes, and respiratory tract. Slide preparation should be undertaken only in well ventilated surroundings!
All of the photomicrographs in the article were taken with a Nikon Coolpix 4500 connected to a Leitz SM-Pol microscope.
For a more detailed explanation of the photomicrographic setup, and the use of compensators with polarized light, please see my earlier article concerning ascorbic acid.
All comments to the author Brian Johnston are welcomed.
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy
UK web
site at Microscopy-UK