Using a very simple technique, you can produce elegantly beautiful patterns which are readily examined with a stereo-dissecting microscope. The only tricky part might be obtaining some Silver nitrate. However, there are some sources on the Internet which will sell small quantities of chemicals and that is ideal for the amateur microscopist who rarely needs more than a very small quantity. Another possibility is a friendly pharmacist who might be persuaded to make up a 1% solution of Silver nitrate for you. Silver nitrate does after all have medical and first-aid uses and is found in styptic pencils which is what you use after a night, like New Yearís Eve, of too many libations, when the next morning, you cut yourself shaving and need to staunch the bleeding.
Next go to a hardware store and buy a small coil of the very thinnest copper wire that they have. Alternatively, if you have an old extension cord around the house, thatís no longer being used, cut of a few inches, remove the insulation, and youíll have a bundle of very thin copper strands. However, make sure that you have a copper extension cord and not one using some other metal.
Cut several pieces of the copper wire, about Ĺ inch in length will do, and keep them in a small flat container with a lid. Place a drop of the Silver nitrate solution on a clean slide and take a pair of tweezers and carefully drop one of the copper strands into the solution. In a few seconds, silver will start to deposit around the strand. This is quite interesting to watch and the process can go on for 2 or 3 minutes, depending upon the amount of copper, the amount of solution and its concentration.
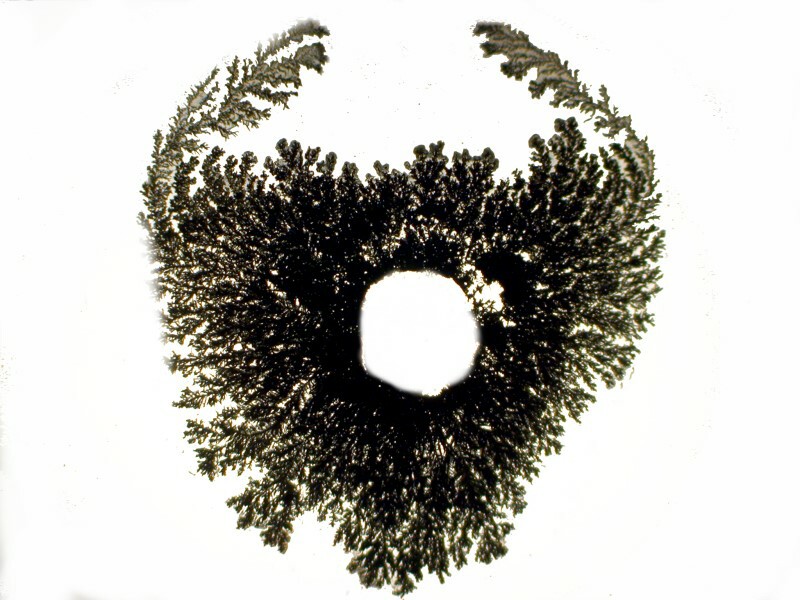
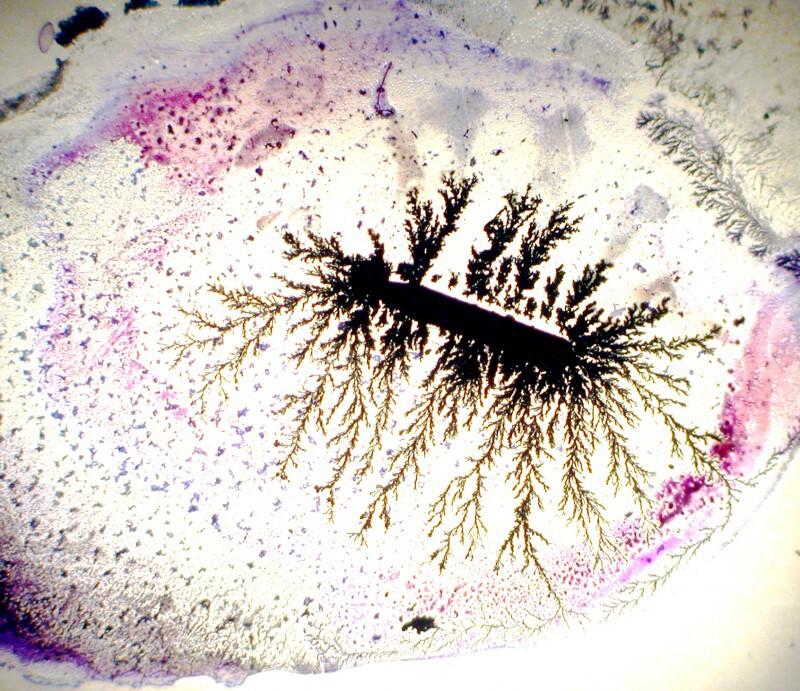
First Iíll show you an image that is very like a tree and then another one which looks like trees here, high in the mountains, where the wind blows almost constantlyĖso, a wind-swept tree.


The size of the copper strand and its positioning and shape will affect the sort of patterns that are produced which can vary in intriguing ways.
For the next image, I took one of the very fine extension cord strands and curled it into a miniature coil which I placed in the center of a drop of solution. The result produced a form that looks rather like a coat-of-arms or an escutcheon. The image after it is the same one after I removed the coil from the slide.

When observing with a typical brightfield configuration, the silver deposits usually appear gray to black in an oxidized form. However, a slight shift of the mirror to change the angle of illumination produces a distinct coppery color. The first image is brightfield, the second is at exactly the same point on the slide; the only difference is the shift of the mirror.
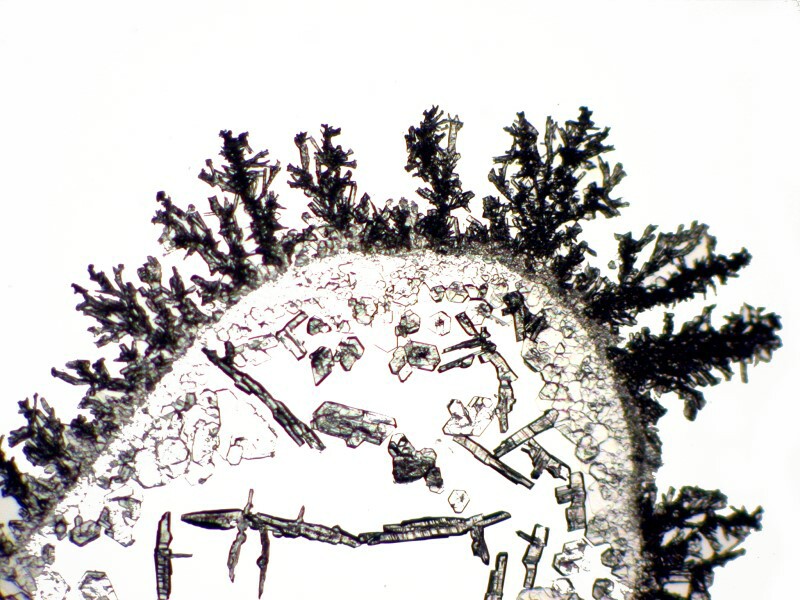

With a bit of experimenting by shaping the copper strand, you can come up with an interesting variety of forms. Iíll show you 3 examples.



Now, if we take a closeup look at a portion of the last image, we can see that there is an elaborate matrix of interlocking crystals which is somewhat reminiscent of the intricate grids in certain glass sponges.

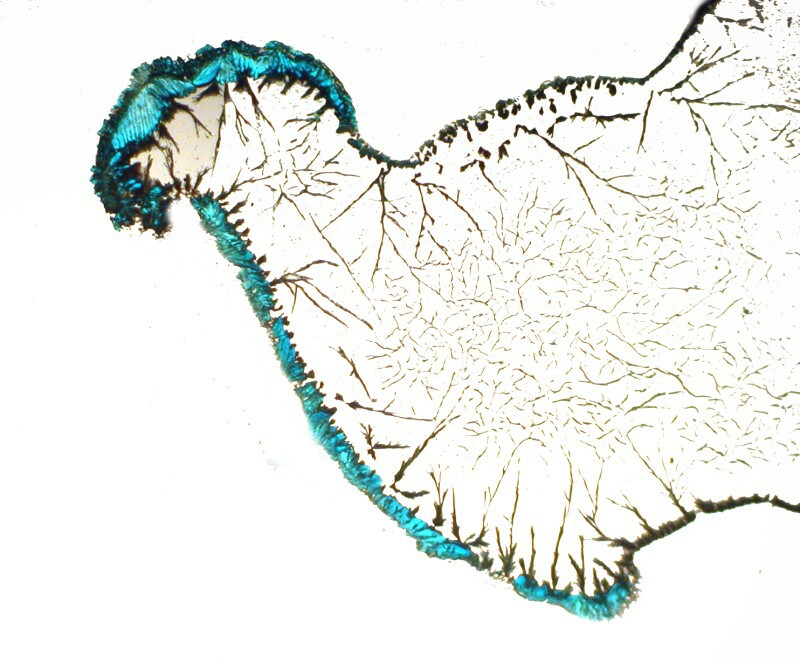
I then decided to try a double dose of copper by using a thin strand, but also adding a drop of copper acetate to the Silver nitrate drop on the slide. This produces some lovely effects. This first image is still very-tree like, but you will notice that in the upper left there is a greenish-blue tint from the acetate.

Here in this closeup, it is even more evident.

Out at the edge of a drop, the Copper acetate has crystallized and there are thin dendrites of silver extending through the central area.
A closeup shows us just how colorful these crystals can be.
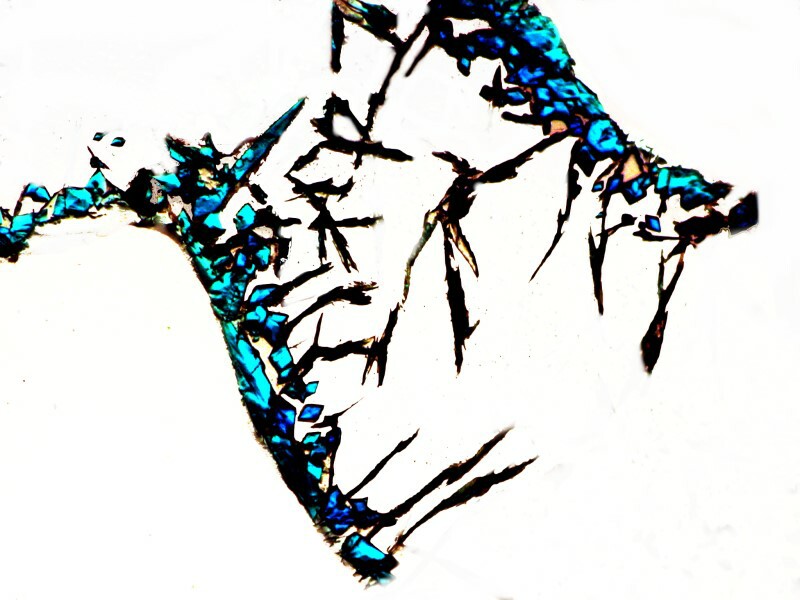
At yet a different location on the slide, we can focus on the silver dendrites and note that in the surrounding area the Copper acetate has created some pastel shadings.

I mentioned earlier that I might try mixing in some stains. I tried 2 and will show you the results here. The first involved placing a drop of Silver nitrate on a slide and adding a small strand of copper and then a drop of Nigrosin. Nigrosin, by the way, is a wonderful stain for the pellicle of protists that can withstand drying after the addition of the stain. In the image below, we will find patterns that are fairly typical of dried Nigrosin even without any additional chemicals, so here it seems that the Copper and Silver nitrate have had little effect.

The other stain was a Trichrome stain which was a proprietary (that is, a secret formula) stain from a biological supply house. It is apparently no longer available as itís not listed in their most recent online catalog. It lends a bit of color, but other than that doesnít seem to have much affect. So, it would seem that, in general, stains are not of much benefit for these experiments, except perhaps to lend a bit of color contrast in some instances.
Dendritic forms are fairly common in nature and you can find an interesting discussion of such in DíArcy Thompsonís classic work On Growth and Form. One morning last month, it was -24 F. when I got up and I noticed frost crystals on the bedroom window. Here are 2 examples of dendritic frost.


Finally, another kind of classic example is the dendrites found in certain types of agates and also on limestone. Iíll show you an example of dendrites on limestone formed by the deposition of Manganese oxide.


Keep experimenting; there are wonderful things to discover all around us.
All comments to the author Richard Howey are welcomed.
Editor's note: Visit Richard Howey's new website at http://rhowey.googlepages.com/home where he plans to share aspects of his wide interests.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
© Microscopy UK or their contributors.
Published in the February 2016 edition of Micscape Magazine.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk .