
The LOMO MBS-10 makes a wonderful instrument for observing minerals, and it is dramatically less expensive than similar instruments. A special photographic attachment is available for it that allows it to be attached to interchangeable lens cameras with the M-42 thread. This is the same diameter as the common T mount fitting, but the thread pitch is, unfortunately, different. Fortunately there are attachments available that allow adapting M-42 to several other camera mounts such as Canon and Nikon. (These instruments are presently for sale in the USA with the designation SF-100, they seem identical to the MBS-10 model.
There are two important hints about the photo attachment. (1.) Periodically a drop of clock oil needs to be placed on the pivots that move the prisms that redirect the light, or they may seize. (2.) The oculars need to be covered during exposures.
The MBS-10 that was used to make the images below was equipped with both the LOMO camera adaptor and the LOMO fibre optic illuminator. The instrument was originally purchased with these items in the late 1990s. Ring light LED illuminators are now available that are less expensive and provide light with a colour quality closer to daylight.
Mineral specimens can be obtained in many ways. One can find many interesting ones by simply picking them up off the ground, especially in mountainous areas. Small specimens can be obtained inexpensively from rock and mineral shows, from dealers, and by ordering them from dealers in remote corners of the world. Many minerals that look rather uninteresting without magnification are spectacular when examined microscopically. (The converse of this statement is often true too.) Some minerals are very rare, and hence expensive, however, because only small specimens are needed for microscopic study, even quite rare minerals can be obtained inexpensively when they are only for microscopic study.
Minerals are more or less pure chemical compounds. Some minerals that occur as extraordinarily beautiful crystals are also important commercial materials for certain metals. The images below are all taken with a Canon digital SLR camera mounted on the MBS-10 microscope described above. Unless specified otherwise, the 1X magnification was used.
Stibnite is Sb2S3. It is the chief source of elemental antimony. It is very dark grey mineral that forms orthorhombic crystals. (The unit cell of orthorhombic is a rectangular box with all three sides having unequal dimensions.) It tends to form long, often spectacular needles. Often the crystals are curved. They have a metallic look. The long axis of the crystals tends to be striated. The image below is of a sample was obtained from a dealer in China.

The following image shows another view of this same spectacular specimen:

The next specimen is a sample of rhodochrosite. Rhodochrosite is MnCO3. Compounds of Mn such as this one are pink to red, depending on the thickness of the specimen. This mineral crystallises in the trigonal system. The material forms very beautiful crystals, but tends to be too soft to use for fabrication of jewelry.

Strontium is widespread in nature, however, minerals involving it are not especially common. Celestite (sometimes called celestine) is SrSO4. It forms orthorhombic crystals. Pure celestite is white, the specimen showed here has an orange cast to it from trace impurities.

One of the world's most beautiful minerals has to be crocoite. Crocoite is a simple compound, PbCrO4. It crystallises in long monoclinic needles. Most of the good specimens seem to come from Tasmania. This is one of only a very few minerals containing the chromate ion.
The specimen shown here is from Tasmania. Note the way the spectacular orange-red crystals lie in all directions.

The next image is of a rather rare mineral, proustite. Proustite has the chemical formula Ag3AsS3. It forms trigonal crystals. The ones on this specimen are not very well defined. Unlike most sulphides it is not opaque, and it is bright red in colour. This mineral is somewhat light sensitive, and should be stored in the dark.
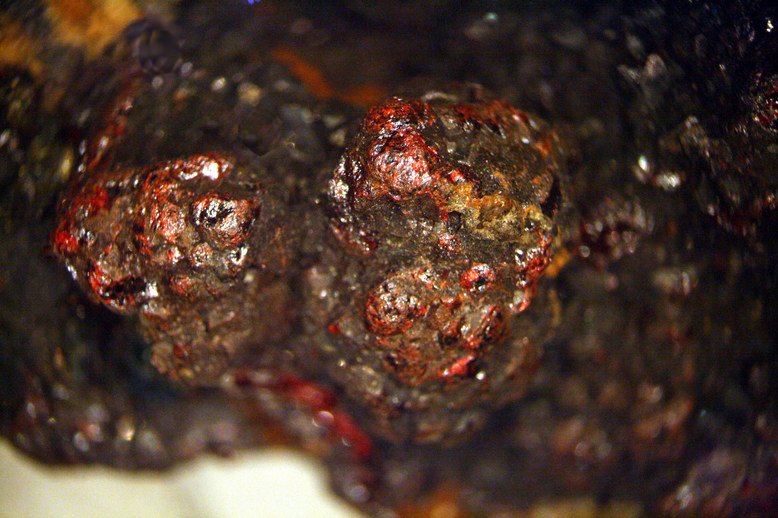
The next image is of another silver mineral, acanthite. Acanthite is one of two forms of Ag2S. It forms monoclinic crystals. Like all silver minerals, this material is quite uncommon, though more common than prousite.

The next image is of vanadinite. Vanadinite has the formula Pb5(VO4)3Cl. It is a major industrial ore for vanadium. It is a somewhat uncommon mineral, but its bright red hexagonal crystals are spectacular. Deposits of it are found in many places in the world. This specimen is from Morocco.
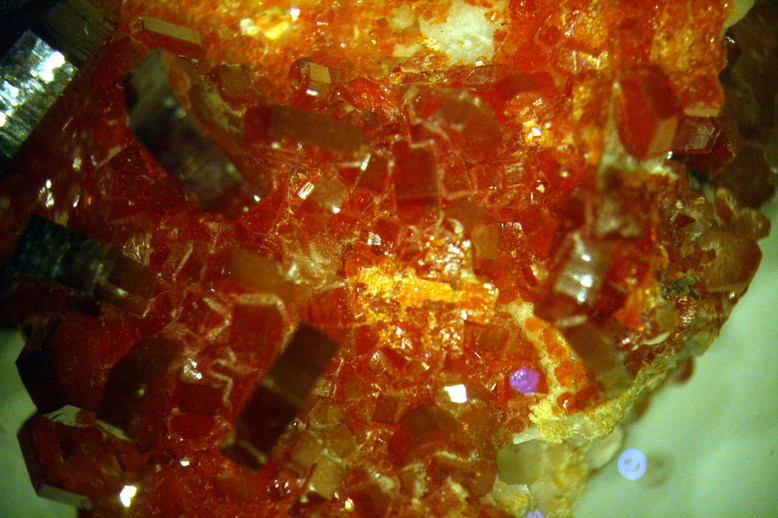
The following image is of a different specimen of vanadinite. Here we are looking down on the square faces of most of the crystals.

There are a few elements that occur in nature as the free element. One of these is copper. Native copper deposits are relatively common in upper Michigan in the USA. Before the development of modern electro refining these deposits were extremely important, because they are rather pure Cu. The image below shows a specimen of native Michigan copper.

The image below shows the same specimen but using the 4X objective.

The mineral pyrite is iron sulphide, FeS2. It has a metallic lustre and a colour very similar to gold, so people often refer to it as fool's gold. It forms cubic crystals.

The following specimen has several minerals in it. It was given to me as a gift by my brother, Terry Pavlis, who is a geologist on the faculty of the University of Texas at El Paso. According to him, the specimen comes from China. It contains garnet, microcline, and quartz. It is a rather spectacular specimen, even though the minerals it contains are quite common.

The next image is of the same specimen, but at higher magnification (4X objective.)

Silicon carbide can form gem quality stones. Natural stones are very small and very rare. Synthetic ones are very expensive too, because they are very difficult to produce. Moissanite is an hexagonal silicon carbide. When cut properly its optical properties are certainly better than diamond. It is very difficult to cut this material into gem stones. The image below shows a specimen of synthetic moissanite cut like a diamond. Only the "snob appeal" of carbon diamonds prevents this material from being used much more extensively in jewelry, as the stones tend to look better than diamonds and are more durable. Were diamonds priced in a free market, they would cost less than these!

The images described here were all obtained with the LOMO MBS-10 stereo microscope. Many minerals are spectacular under this type of instrument. Minerals that form small crystals may require higher magnification than can be obtained by these instruments.
All comments to the authors via Robert Pavlis are welcomed.
Microscopy UK Front Page
Micscape Magazine
Article Library
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK