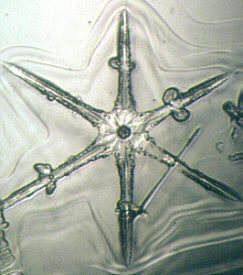
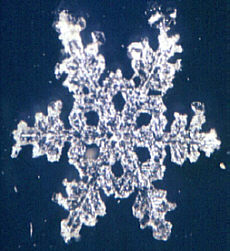
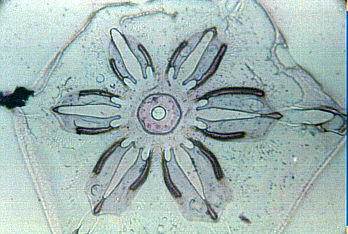
You may be surprised to know that there is a technique that will allow you to make plastic "casts"of snowflakes so that they can be studied indoors. You may think this is a relatively new, high tech method, but in fact it was developed over 50 years ago by Vincent J. Schaeffer1. It has been detailed in photography, microscopy, and meteorological magazines/books over the years, but many amateur microscopists are still unaware of it. The method really is quite simple. Prepare a 1% solution of polyvinyl formal in ethylene dichloride. <Please read footnote and safety notice below>. Keep the solution in a small vial at below freezing temperatures. Using a cotton swab, swab a large drop onto a cold microscope slide and either let snowflakes fall directly in the solution, or transfer flakes to the solution with the aid of a small artist's brush. Leave the slide in a cold, protected area for 5-10 minutes and VOILA you will have permanent replicas of snowflakes.
I started snowflake collecting about 25 years ago, mainly to have something to do in the winter when microscopic specimens were not available from frozen ponds and streams. It became such an interesting diversion, that now it occupies most of my "free time" with the microscope. Zeeland, MI is about 15 miles inland from Lake Michigan and we tend to get lake effect snow during most of the winter, making this area an "ideal" place for snowflake collecting.
Once you become proficient, you will be surprised to find how "rare" a well-formed snowflake is. Most storms produce distorted specimens that are not worth saving. Snowflakes may form 5 miles high up in the atmosphere and during their perilous journey to the ground, updrafts, downdrafts, and wind in general all take their toll. Nevertheless, there are always two or three snowfalls during a winter season when conditions are perfect-no wind, very cold temperatures, light snowfall—that will allow you to get good specimens. The trick is to be ready! Happy hunting!
Comments to the author James Benko welcomed.
Image gallery by the author.
|
|
|
|
|
|
Further Reading
Schaeffer, V. J., "Snow and its Relationship in Experimental Meteorology", The Compendium of Meteorology, American Meteorology Society, Boston, MA, pp 221-222, 1951.
In this article Schaeffer describes the replication technique as follows - "In 1941, a method was devised by the writer for making permanent replicas of snow crystals. The technique encases a snow or frost crystal within a thin plastic film, which, as it forms, makes an exact three dimensional impression of the surface features of the crystal. The replica solution, consisting of one to three parts of synthetic polyvinyl formal dissolved in 100 parts of ethylene dichloride, readily wets an ice surface. By capillarity and fluidity, it rapidly covers any ice crystal which comes in contact with it. The solvent evaporates in five to ten minutes after which the slide (glass, cardboard, etc.) bearing the samples may be warmed above freezing. Upon melting, the water molecules evaporate through the thin film leaving a hollow shell which reflects and scatters light in a manner quite similar to the optical properties of the original crystal."
Micscape safety note: As the author reports this is a successful and traditional method for encapsulating snowflakes with excellent results, but unfortunately the technique can't be recommended nowadays to the hobbyist. Ethylene dichloride (1,2 - dicholoroethane) is classed as Toxic/Very Toxic and a known/suspected carcinogen. If the solution is made up on your behalf, the Material Safety Data Sheets for these chemicals should be provided by the suppliers, consulted and the appropriate precautions taken before use.
Footnote: The author James Benko also remarks: 'As a chemist I still have access to this chemical at work, but I agree it may be hard for an amateur to get a supply, since you cannot buy it at your local hardware store. Still it is the best solvent for this type of work. I have tried other solvents, but they do not work. The only thing that comes close is chloroform but also hard to come by. One approach that still may work is to use hair spray or acrylic lacquer spray. If it is kept cold and then sprayed onto a slide, snowflakes are transferred to the wet solution on the slide, then they are sprayed again and allowed to dry. I have had only partial success with this method. I may not be doing it right, but if this is all one has, one can keep trying to get the exact conditions for making a good prep. I have seen the Schaeffer method repeated in many science journals, photography books, etc. and they always recommend a 1-3% solution of polyvinyl formal in ethylene dichloride.'
The author and Micscape would be interested to hear of any experiences with safer solvents for polyvinyl formal (or polyvinyl acetal) or techniques which are accessible to the amateur for encapsulating snowflakes. Another interesting approach for the hobbyist to encapsulate snowflakes uses clear nail varnish (see 'Snow Crystals' by Arthur Burton in 'Microscopy and Analysis', January 1991, page 7).
The web site Ecotarium also describes a procedure for making plastic snowflake replicas using 'Workable Fixative' (available from art shops to 'freeze' areas of artwork) .
Disclaimer: This article is offered in good faith by the author. Neither the author or Microscopy-UK, Micscape or Onview.net or any of its administrators or contributors assumes any responsibility for damage to persons or property incurred by using the chemicals described.
Also see the Micscape article by Ed Kinsman on 'Photographing Snowflakes'.
James Benko writes on painting snowflake replicas in the November 2000 Micscape.