It's very satisfying when a number of interests cross fertilise each other and would like to share a recent example. As a retired industrial chemist seeking to keep some rusty brain cells active I've become fascinated with the wonderful variety of chemicals that plants and animals exploit for various purposes. The more complex the better (if they are not macromolecules) as enjoy building them with molecular model kits. A new book that am enjoying reading is Most Delicious Poison. From Spices to Vices - the Story of Nature's Toxins by Noah Whiteman (Pub. OneWorld).
In Chapter 3 on terpenoids he discusses birch trees—their importance to indigenous peoples and chemical interest. The compound betulin is responsible for the distinctive white bark and he notes can form up to 35% of a birch extract. Rubbing the bark can readily remove this as a powder. The compound has a number of roles for the tree including reflecting the strong sunlight of winter in the northern climes where it grows. The compound and its derivatives may also have potential medical uses. (A Google search shows that it can be bought pure as a supplement with various claims of its efficacy.) The author also notes that it was one of the first chemicals to be isolated from a plant. The Wikipedia entry for Betulin revealed that it was first extracted by Johann Tobias Lovits (Lowitz) 1757-1804. He was a German-Russian chemist who established a number of laboratory practices (see refs.) Of particular interest to the microscopy enthusiast is the Wikipedia note that 'He examined crystallization from supersaturated and supercooled solutions, noting the application of seed crystals.' Other biographies (see refs.) note that he used a microscope for these studies and even made models of different crystal morphologies observed.
I was keen to walk in the footsteps of this pioneer of crystal studies. The birch native to England is silver birch Betula pendula and very common locally. It is one of the first trees to colonise open areas. A local abandoned recreation ground that I visit on my walks illustrates this as a clump of birches are growing on the former football pitch. This bark does not peel but in a new housing estate close to my home, the paper birch Betula papyrifera has been widely planted. Peeling bark can readily be removed without damaging the tree.
The Wikipedia entry notes that betulin is soluble in alcohol so steeped finely cut bark in pure isopropanol (readily available to the public unlike ethanol). Various conditions were tried to encourage slow growth of crystals from the clear liquor including putting slides with a drop of the solution in the fridge both covered and uncovered. I've had no success so far at growing large crystals, most slides crystallised rapidly to form dense microcrystals. Open evaporation in a cavity slide has given the most discrete albeit small crystals so far, see below. I'd be interested to hear from readers who try to crystallise betulin and if any better success. The liquor of course may contain a number of chemicals including betulin which may or may not complicate good crystal growth.
So some current reading brought together my interest in Nature's chemistry set, model building, a love of the outdoors and local trees, crystal studies and learning about a pioneer of such studies. Perhaps we should make Johann the patron saint of crystal studies using the microscope!


A paper birch tree on a local housing estate and a close up of the trunk showing the peeling bark.
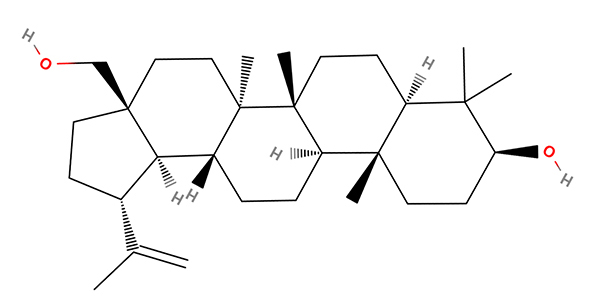
The skeletal formula for betulin created using the online app MolView which as well as being
useful to draw molecules can download molecules as in this case from a database.

A molecular model of betulin built using the molymod® system, in this case with the intermediate length bonds.

A view of a bark peeling under the stereo. A razor blade was used to scrape an area to show the ease of releasing the betulin as a white powder.
The lower left still has a covering of betulin retaining its white colour.
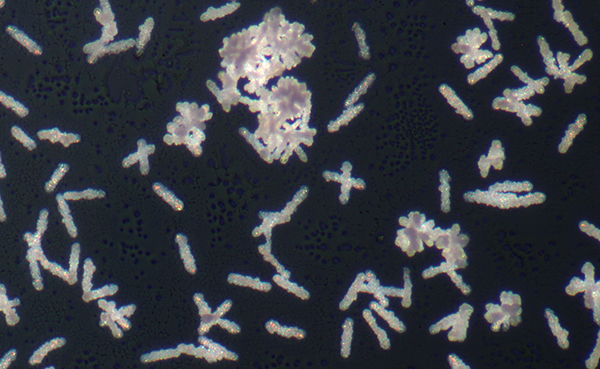
This is the best crystallisation that have achieved to date from isopropanol allowed to evaporate in a cavity slide to provide a solution depth for a slower crystallisation.
Zeiss 6X planachro, transmitted with crossed polars. The morphology is reminiscent of some sponge spicules.
Comments to the author David Walker are welcomed.
References sourced from the Wikipedia entry for 'Johann Tobias Lowitz'
Leicester, Henry M. (1945). "Tobias Lowitz—Discoverer of basic laboratory methods". Journal of Chemical Education. 22 (3): 149-151. Link to open access journal on the Internet Archive site.
Figurovsky, N. (1973). "Johann Tobias Lovits (Lowitz).". Dictionary of Scientific Biography. Volume 8. Charles Scribner. pp. 519–520. Link to open access volume on the Internet Archive site after Borrowing for One Hour option.
Priesner, Claus (1987). "Lowitz, Tobias". Neue Deutsche Biographie (in German). Vol. 15. pp. 259–261. (Site's English translation.)