How does a hot toasty sandwich of Gruyere, ham, and watercress sound? A very tasty melt indeed, but we’re going to be talking about very different kinds of melts. We’ll be looking at crystal melts on slides with polarized light. The slides were all prepared using low heat and selecting only substances that had low melting points and which would not produce any dangerous or noxious vapors.
By heating and melting crystals, one can get some quite different results from those obtained by using the technique of just allowing solutions to evaporate on the slides. In preparing melts, it is important to try to get a thin and uniform distribution of the substance(s) across the surface, otherwise the result is too thick or results in clumping. Both such consequences are highly undesirable for photomicrography. It turns out that sometimes melting a slide more than once can produce quite interesting results that one hadn’t anticipated and are virtually impossible to predict. In this discussion, I will show you a few remelts and will indicate them as such, but unless otherwise noted, the images will be of a single process of melting.
If you have read any of my previous articles on crystals, you will know that I have a prediliction to give descriptive (and sometimes rather eccentric) titles to some of my images, but in this essay, a fair number of them will simply be labelled “Abstraction”, although there are a few cases where I couldn’t resist getting “creative”.
Let’s begin with 2 abstractions; both of these are from a mixture of Tartaric acid (which you can often find in kitchen cabinets) and NMN (nicotinamide mononucleotide) found in small quantities in a variety of fruits and vegetables and is sometimes used as a dietary supplement. I like these two images in part because they suggest a kind of flow or motion.
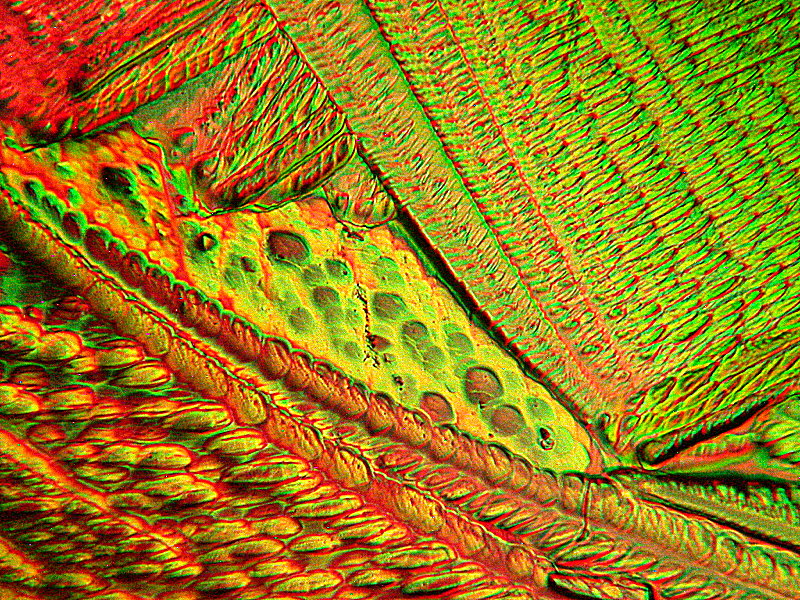
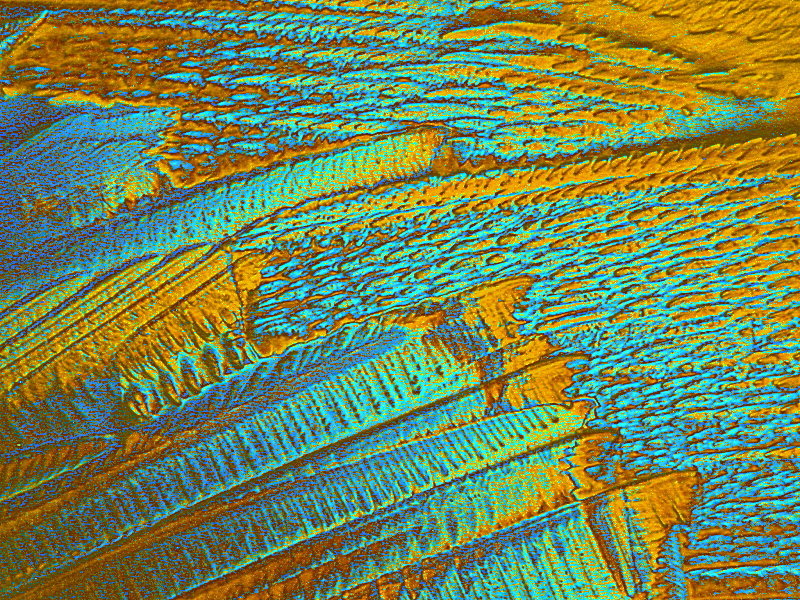
Next, another abstraction and then an image which I call “Leaf”. They are from the same slide which is a mixture of Tartaric acid and Inositol which is a carbocyclic sugar found in various mammalian tissues including the brain. It occurs naturally in corn, beans, and other high fiber foods and in citrus fruits and some melons. These images were photographed from the same slide and which is a remelt.
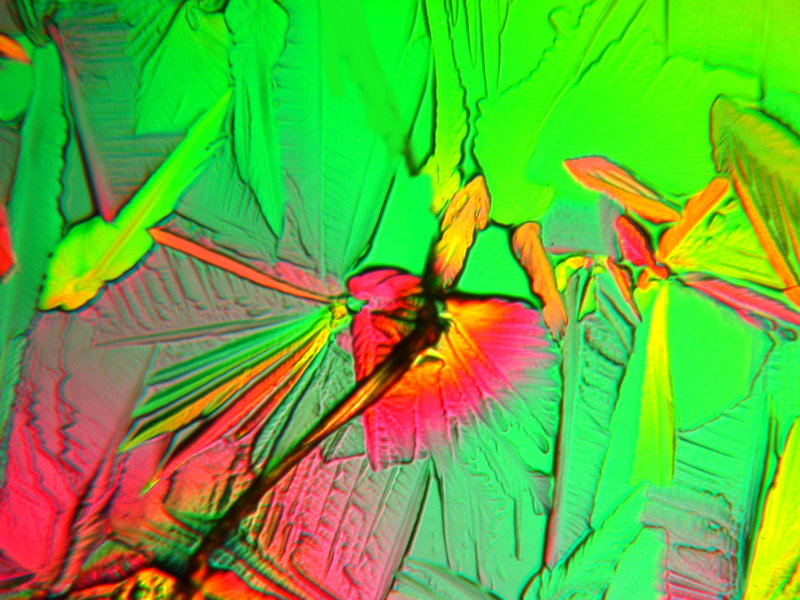
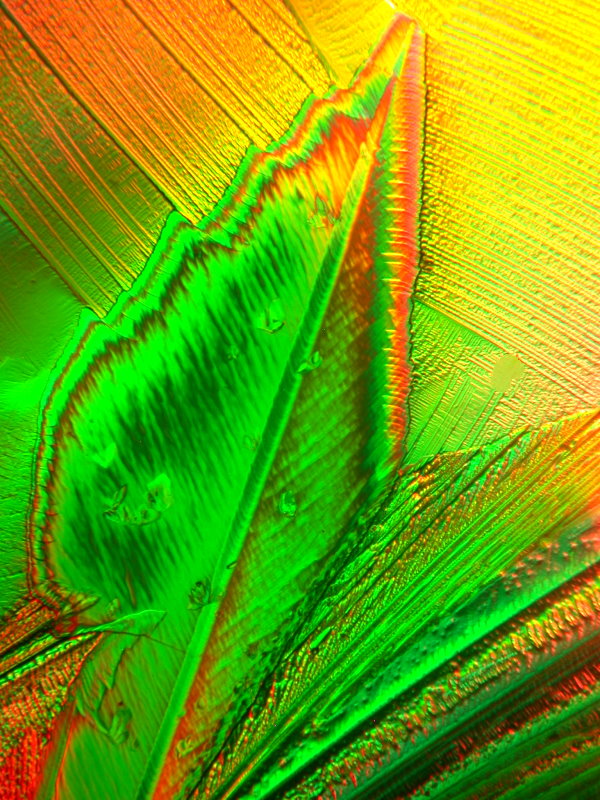
The next 2 images are a melt of Urea, Citric acid, and Copper Acetate. The first one is an abstraction in which the large rather blocky forms appeal to me as being forms one might find in a sculpture. The second on is an image of the “Famous Tower of Bacon”. (Sometimes, I just can’t help myself.)

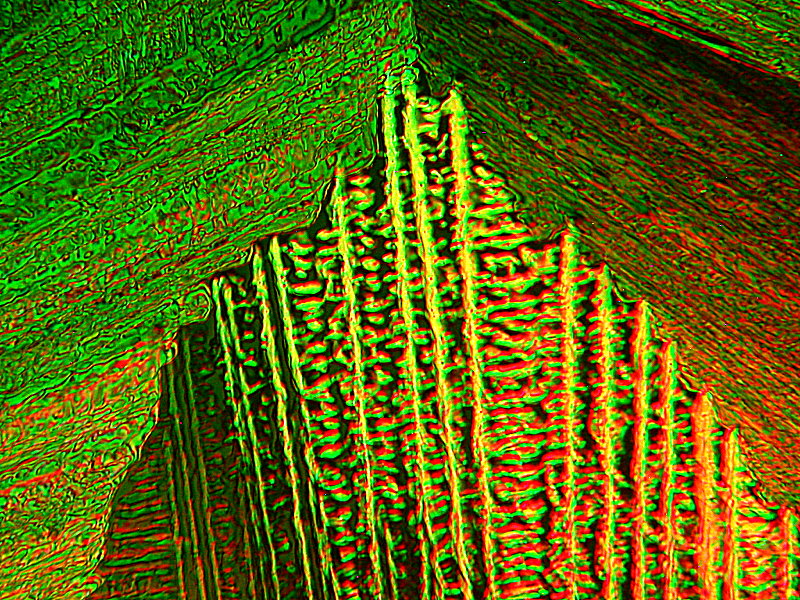
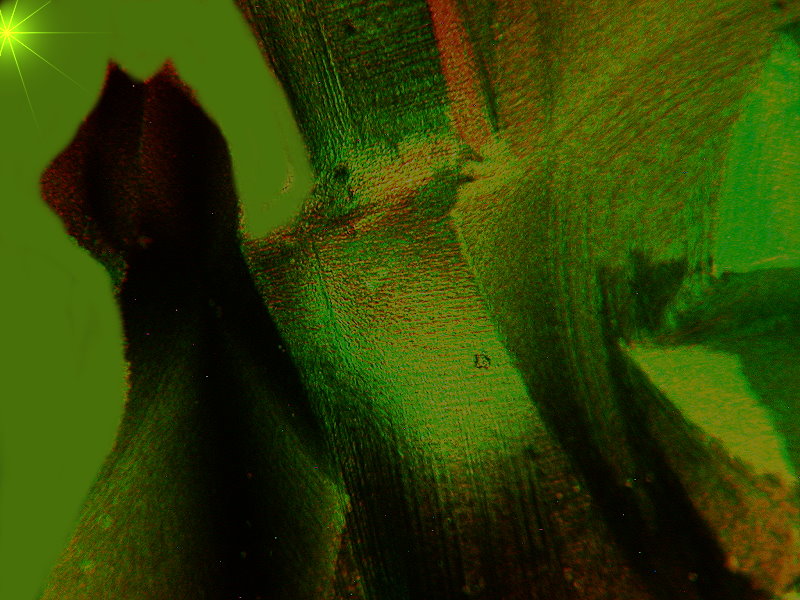
Be warned, the next 2 images also have fanciful titles. They are both from the same slide and yet radically different in character. The slide is a melt of Urea, Ascorbic acid, and Sodium Silicate, which is also known as “Water Glass” and in the days before refrigeration was widely available, eggs were soaked in a solution of “Water Glass” to seal and preserve them.
First I present to you: “Cat by Starlight”. Here I did a tiny bit of computer graphics augmentation, namely, the little star up in the left corner.
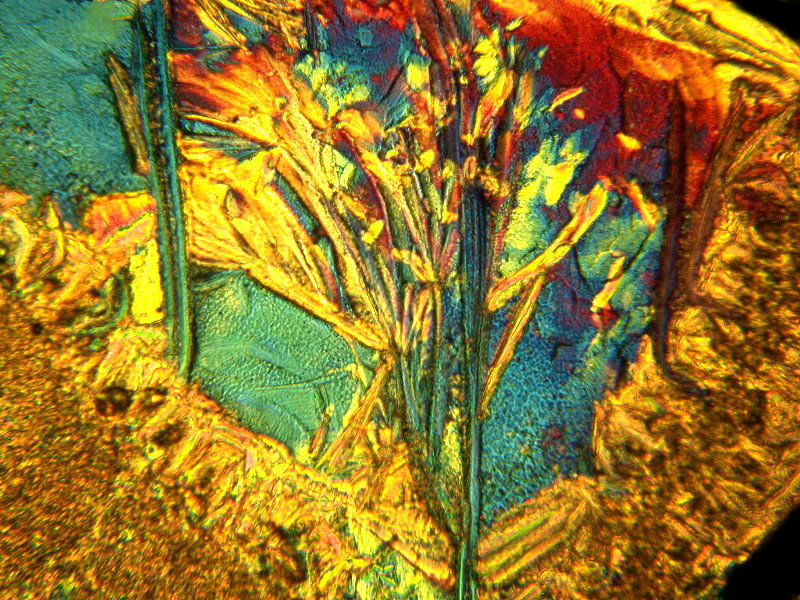
The next one I simply titled: “Lilies”.
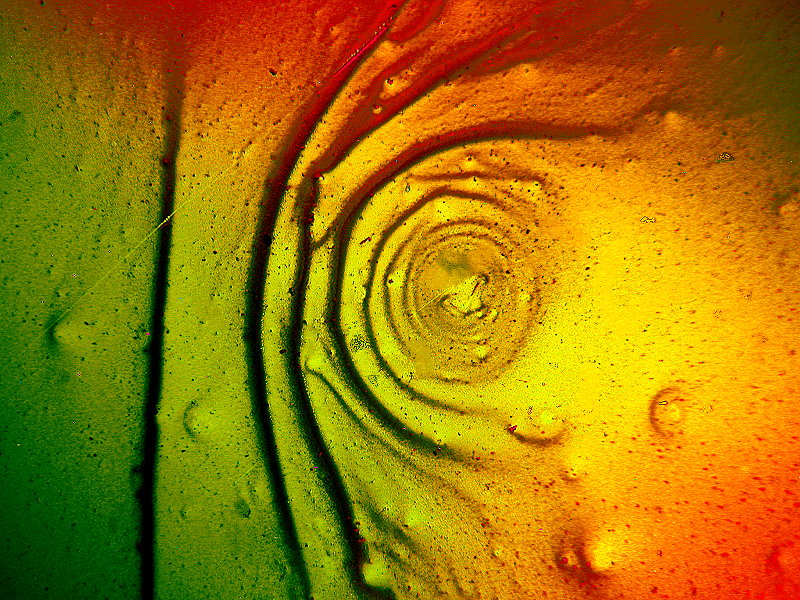
Many of us have been subjected to Menthol cough drops which our loving parents inflicted on us when we were young and had bad colds. Menthol is an organic compound which is derived from the oils of various mint plants. My wife, when she still smoked, indulged in menthol cigarettes. Fortunately, they have now been banned in this country. Menthol has a mild anaesthetic effect and smokers of menthols thus tend to inhale more deeply thereby ingesting larger quantities of toxic substances.
The following image is a melt of Menthol crystals blended with a biological stain, Orange G. I have titled it “Mudpot in Yellowstone”.
Potassium salts frequently produce very interesting results for the polarizing microscope. This next image is a mixture of Potassium bromide, Urea, and Ascorbic acid. I have titled it: “Black Hole Eating A Cosmic Plume”. Perfectly obvious, I should think.

Above, I mentioned that Potassium salts often produce interesting results and the same applies to Copper salts. I am especially fond of Copper acetate. It has lovely pale blue color and tends to produce long, jagged lance-like structures. On occasion, I get an image which tickles my curiosity and I wonder what would happen if I subjected it to a bit of play with computer graphics and, in particular, the function in my program called “creative warp”. The 2 images below are from a slide melt mixture of Copper Acetate, Urea, and Ascorbic acid. The first has been subjected to alteration through “creative warp”, but the second has not and if I were, heaven forbid, in a fanciful mood it might be called “We Are Watching You.
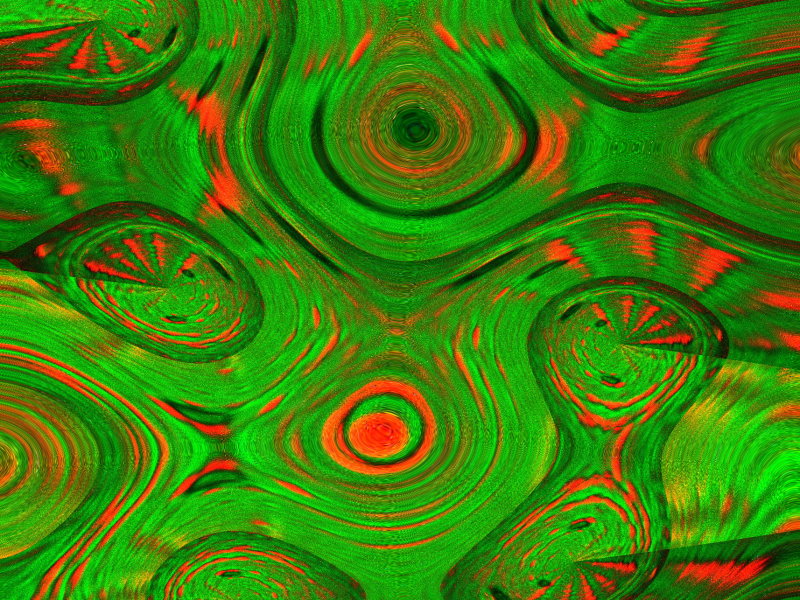
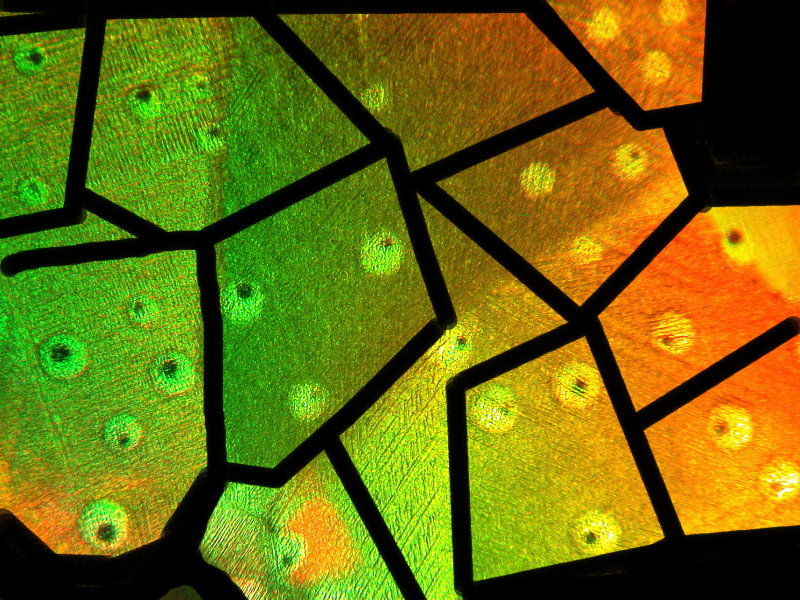
Once again, 2 images in a combination with a Copper salt–Copper acetate and Benzoic acid. The first one is titled “Defective Nuclear Missiles” and the second is “Coral Growth”.


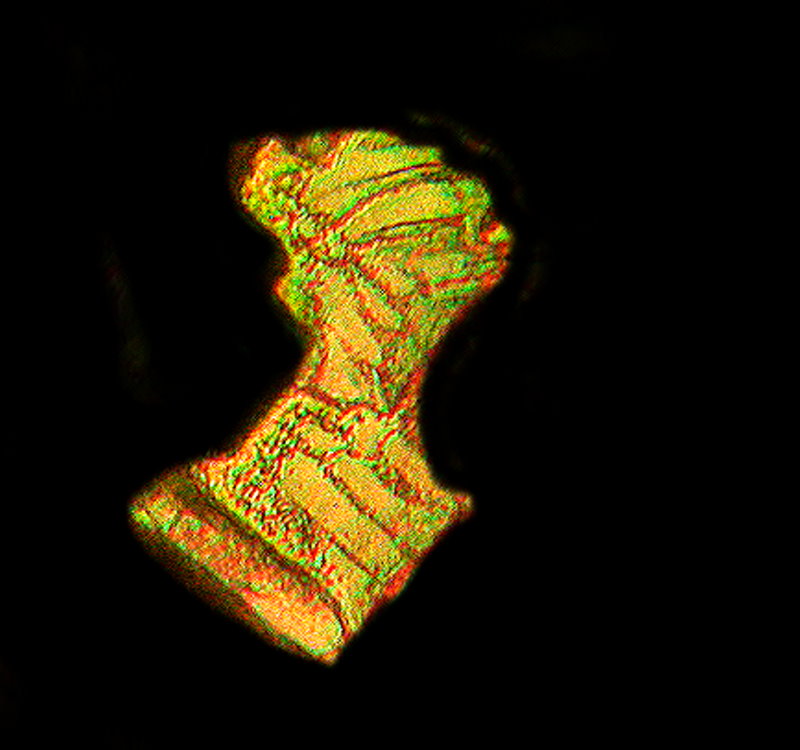
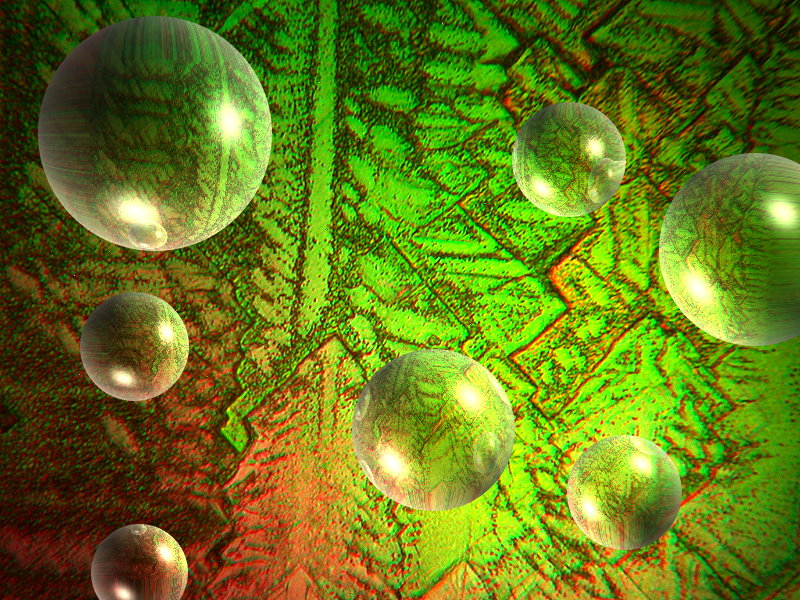
Again with the Copper; I told you it was one of my favorite crystal salts. Copper acetate and Urea melt. The first is titled “Marble Bust” and the second has had a little computer graphics surgery and is titled “Spheres”.
Just for variety’s sake, back to a Potassium salt; Potassium Bicarbonate and Urea melt. The first image is surely a fish from the abyss. Interestingly the second image is the same fish to which I applied the computer function of “creative warp” and the 4 forms there rather remind me of shuriken or Japanese “throwing stars” used by Ninja warriors.
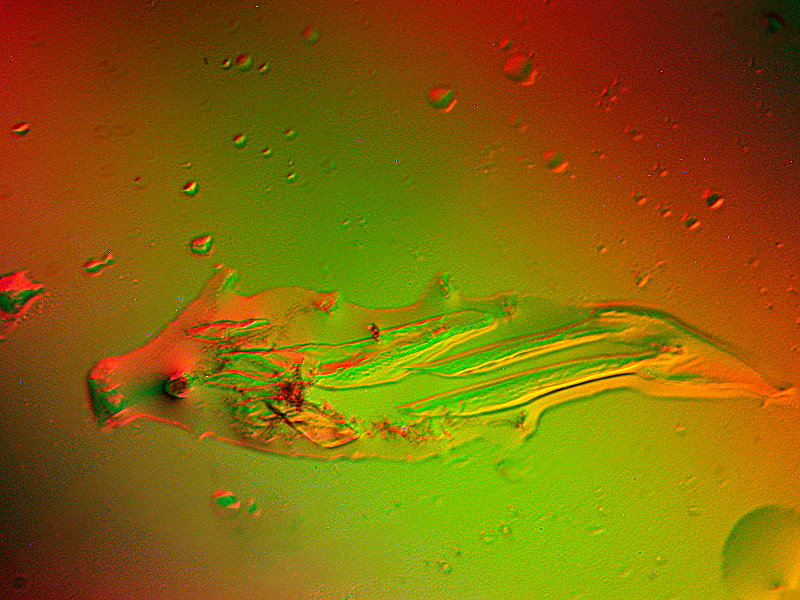

Now we’re back to abstracts and this time with a different salt, namely, Strontium chloride mixed with Urea. Although these are photographed from the same slide, the character of the images is radically different.
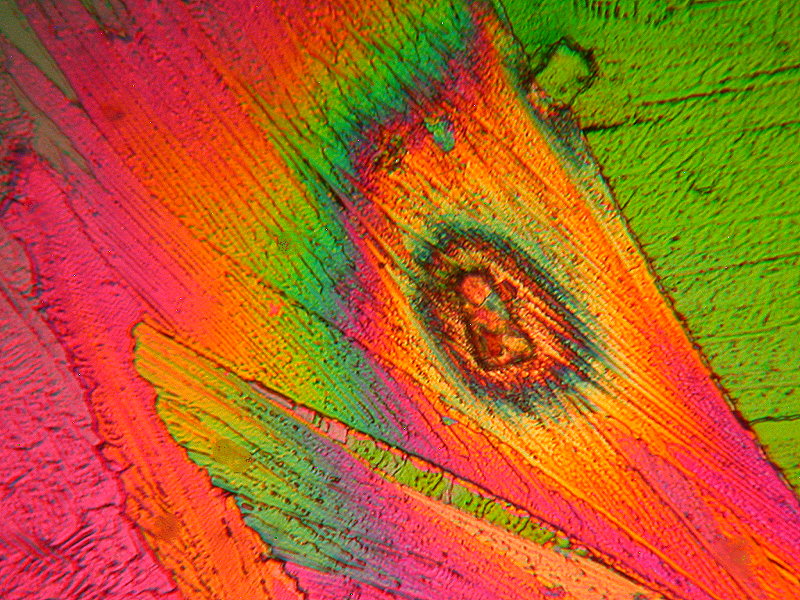
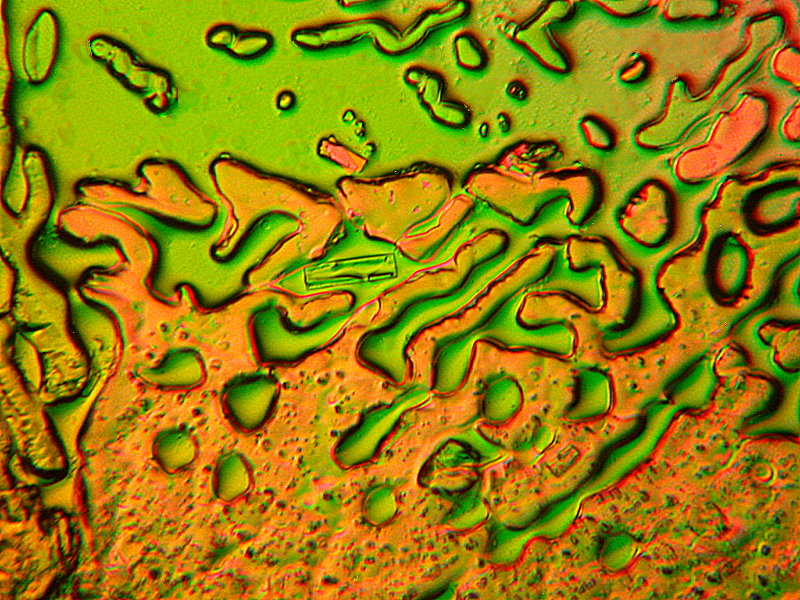
Again, a couple of whimsies and these are relatively simple melts of Ascorbic acid. The first is “Hot Air Balloon in the Form of a Clown” and the second is a perhaps Daliesque image titled “Eyes on You”.
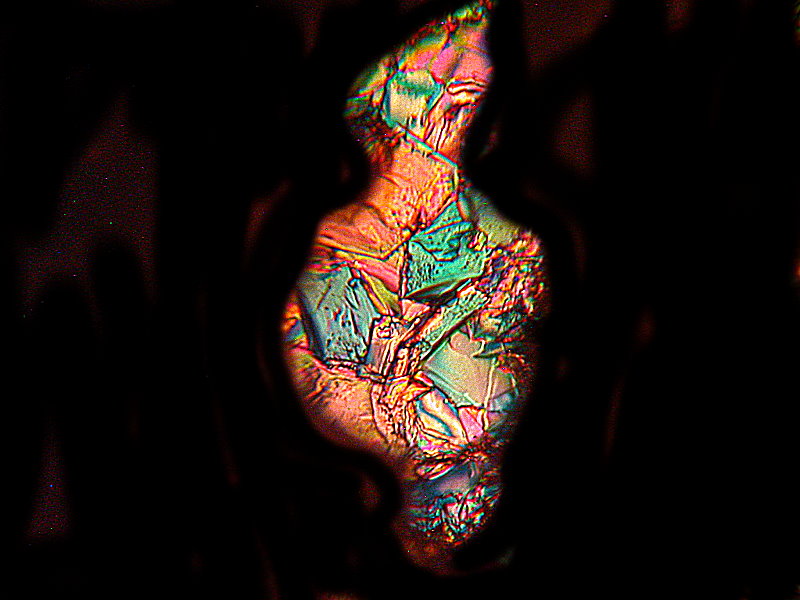
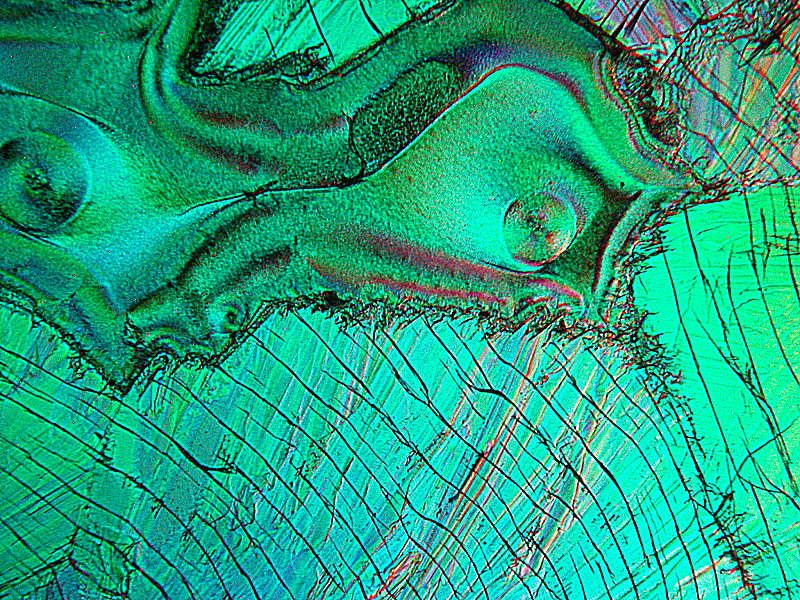
Finally 2 more abstracts and 2 titles images, this time a melt of Caffeine and Vanillin. What especially strikes me here is again that these occurred on the same slide and yet are so visually different.
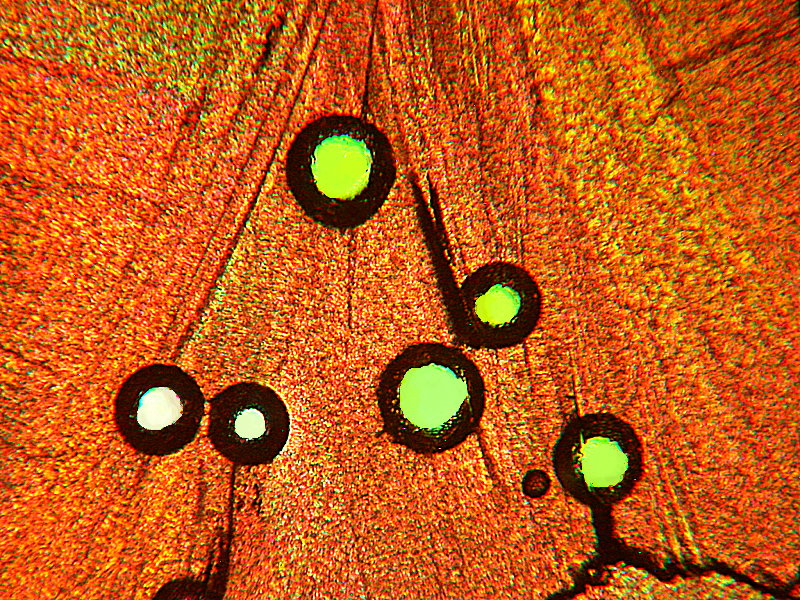

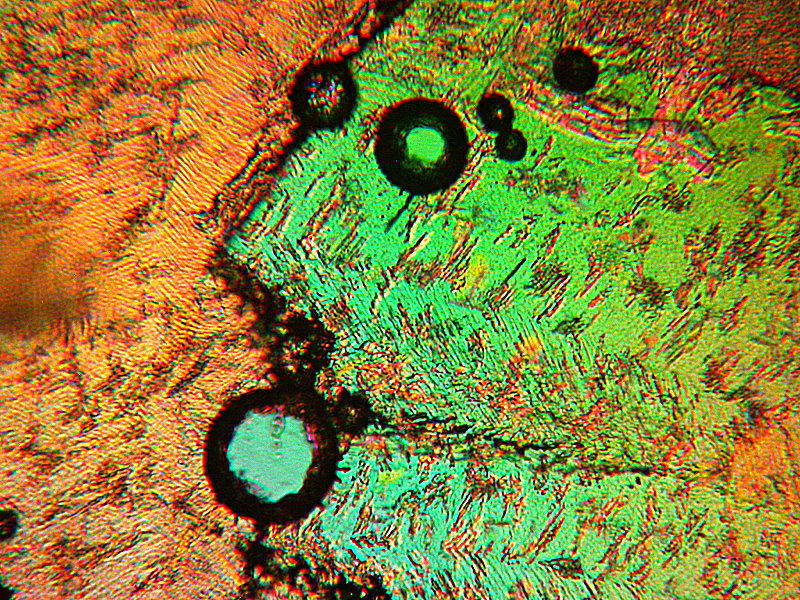
Next “Fish Blowing a Bubble” and “Flower”.

I hope you enjoyed this brief journey into the realm of crystal melts and that you might feel an urge to experiment but, please, always with care and caution.
Acknowledgment
I wish to thank Sawyer Winn, a student assistant, who has prepared many of the slides for these images and for the two previous articles on crystal images as well. We tried a wide range of combinations and were able to achieve some rather good results.
A Plea
Micscape is a wonderful place to try out and share ideas and it is open to amateurs and professionals alike. Please consider sending notes, images, articles, or even a picture of your favorite slide telling why in just few sentences, or ten pages, if you like. Dave Walker is a wonderful editor who is very helpful if you have questions about format, organization of an article, or virtually anything that you might want to submit. Micscape depends upon its contributors to help stimulate interest in microscopy and encourage both young and old to investigate the endless mysteries of Nature. So, please do send lots and lots and quit putting it off and forgetting about it.
All comments to the author Richard Howey
are
welcomed.
If email software is not linked to a browser, right click above link and use the copy email address feature to manually transfer.
Editor's note: Visit Richard Howey's new website at http://rhowey.googlepages.com/home where he plans to share aspects of his wide interests.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
© Microscopy UK or their contributors.
Published in the December 2021 edition of Micscape Magazine.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk .