In the past, I have often presented a lot of crystal images of Ascorbic acid (Vitamin C), but this time I decided to show you a variety of images, some of which include Ascorbic acid, but many of them are mixtures of substances without Ascorbic. Some of these are quite striking and the forms are very different from the characteristic disks and Maltese cross forms in Ascorbic. All of these image were taken using polarized light.
This is a combination of Alkaseltzer and toothache medication. Sometimes I also use a generic form of Alkaseltzer and the results can be somewhat different. Alkaseltzer does contain some aspirin so, that is a factor in the composition of the crystal as well. The toothache medication in this and in other instances in this album is generic. I have also at times used Anbesol and again this can make some difference.
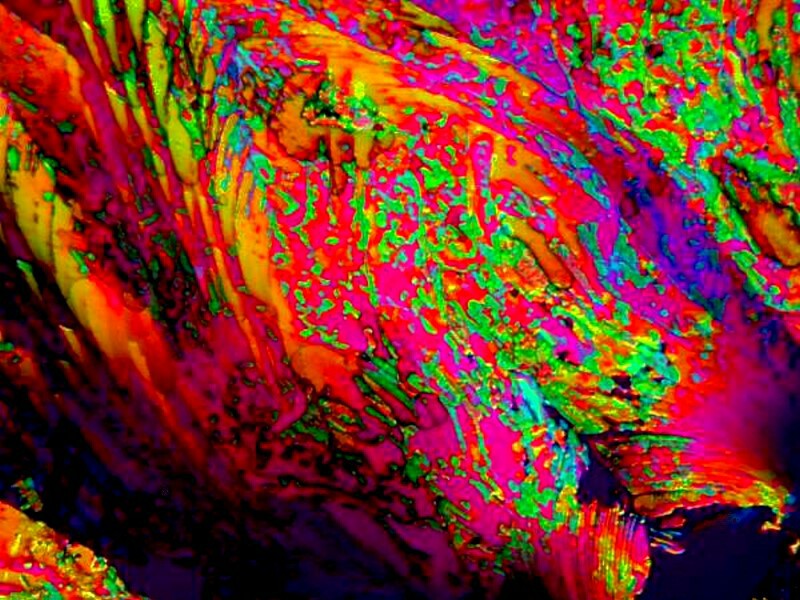
Toothache medications tend to produce very vivid colors in combination with many other substances. Below is a mixture of toothache medicine and codeine sulfate. I used to take this for migraine headaches, but I was beginning to dislike the side effects and quit. I still had a few tablets left, so naturally they went to my lab for crystal making.
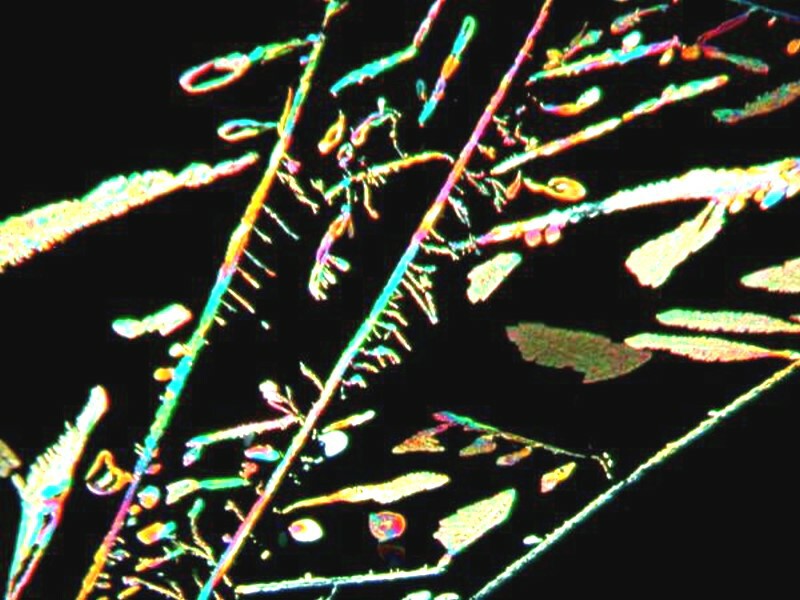
While weíre dealing with toothache medicine, Iíll show you 3 images of just the medicine alone, not mixed with anything else. The first 2 again show vivid color, whereas the third, interestingly, takes on a pastel character with odd little branching structures that remind me of some of the paintings of Paul Klee.


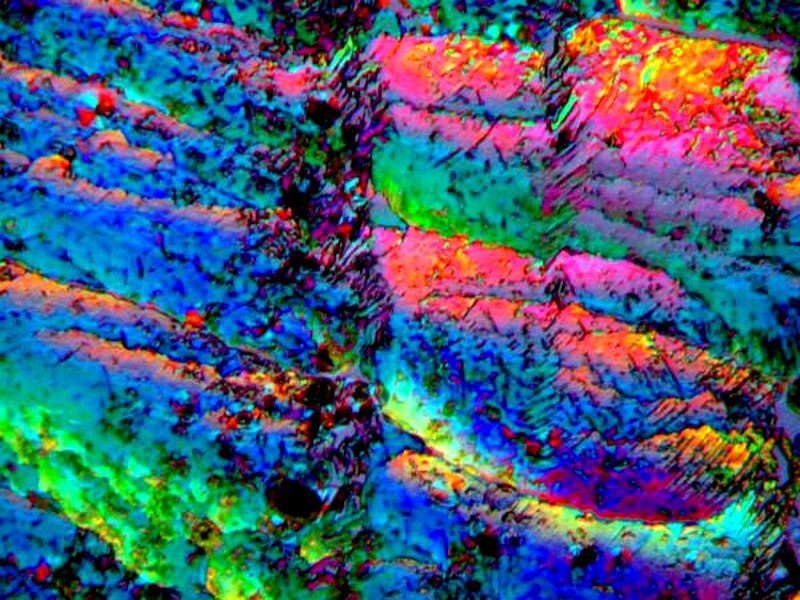
The next 2 are toothache medication again mixed, this time with Magnesium sulfate or Epsom salts. I use Epsom salts bought at the drug store which may have some impurities, but certainly makes for interesting crystals.
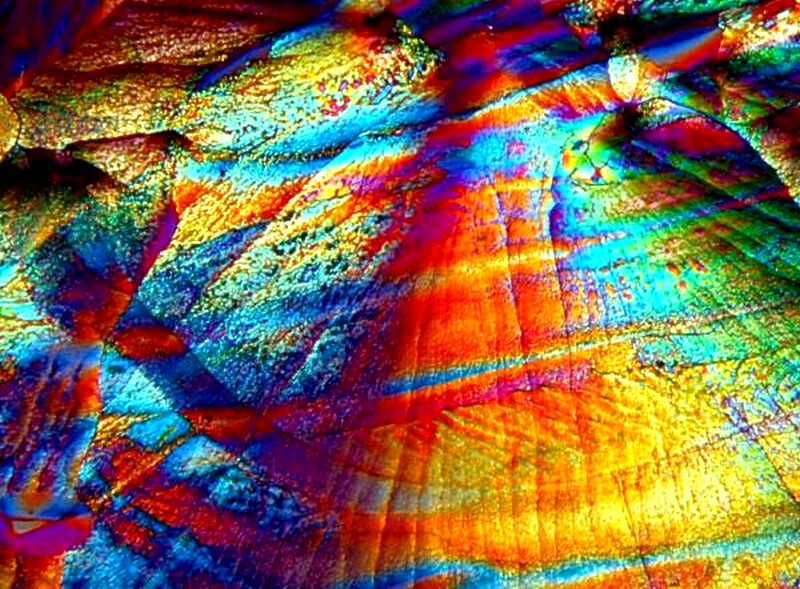
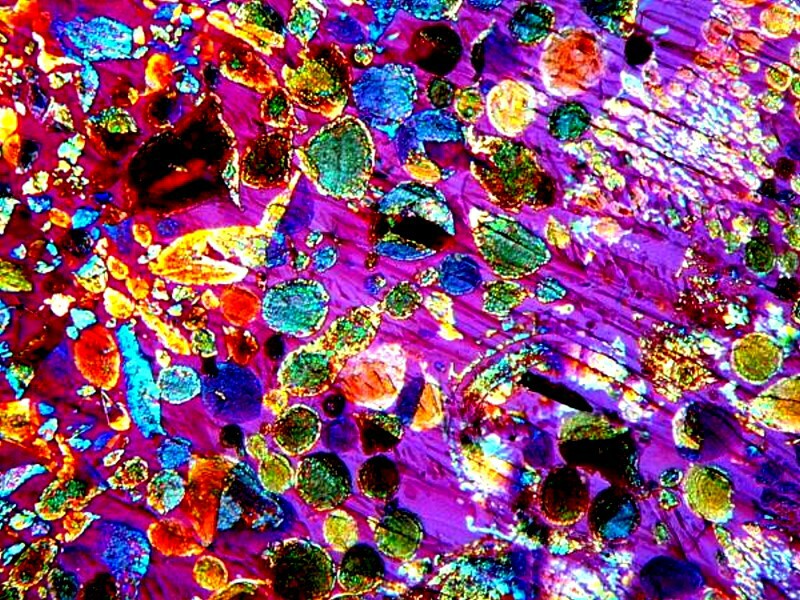
So while weíre on the subject of Epsom salts, Iíll show you some other images. Epsom salts are a delight to work with because they almost always provide interesting forms. By themselves, the produce quite distinct, smooth granular forms. Iíll give you 3 examples here.
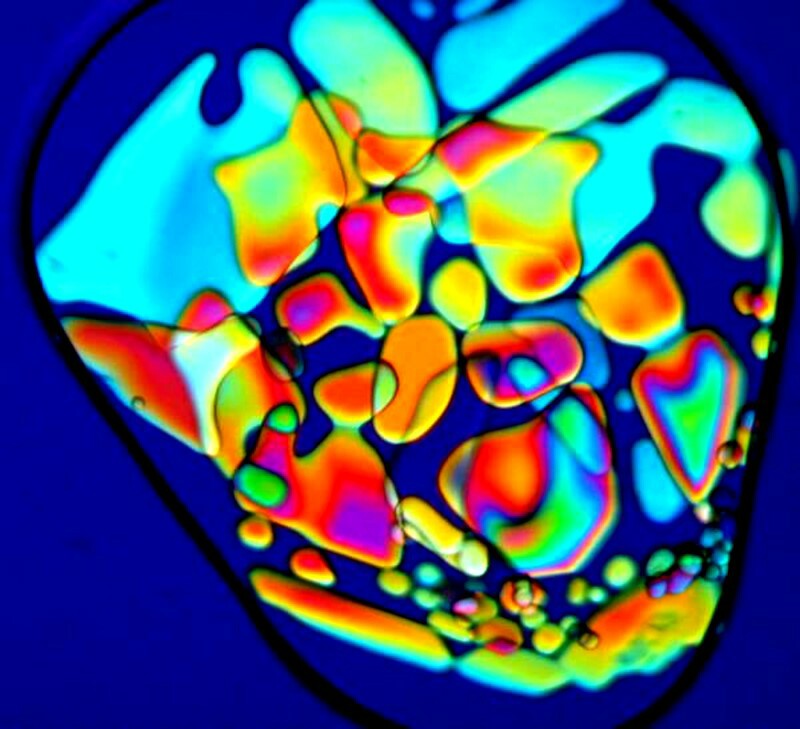
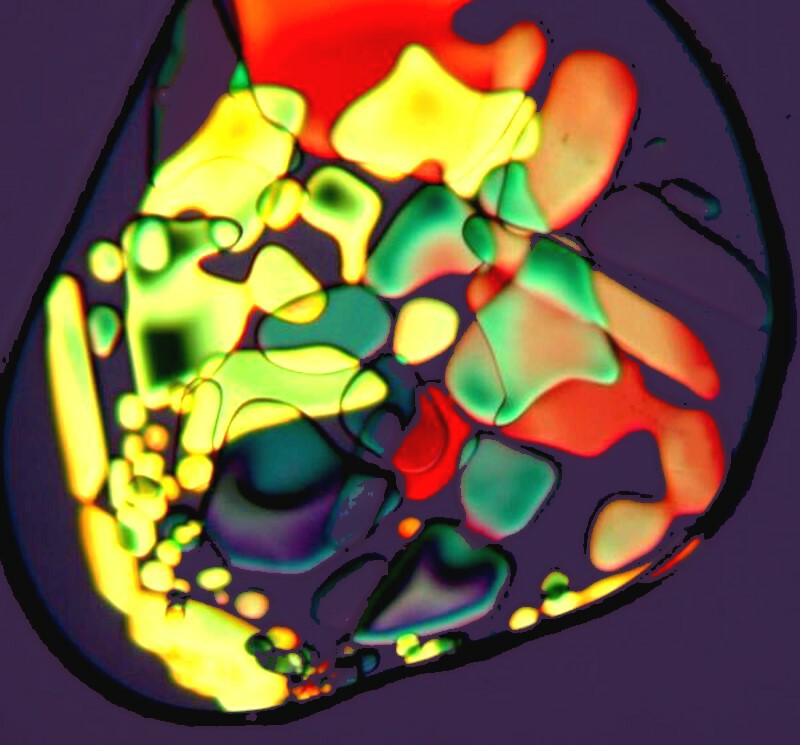
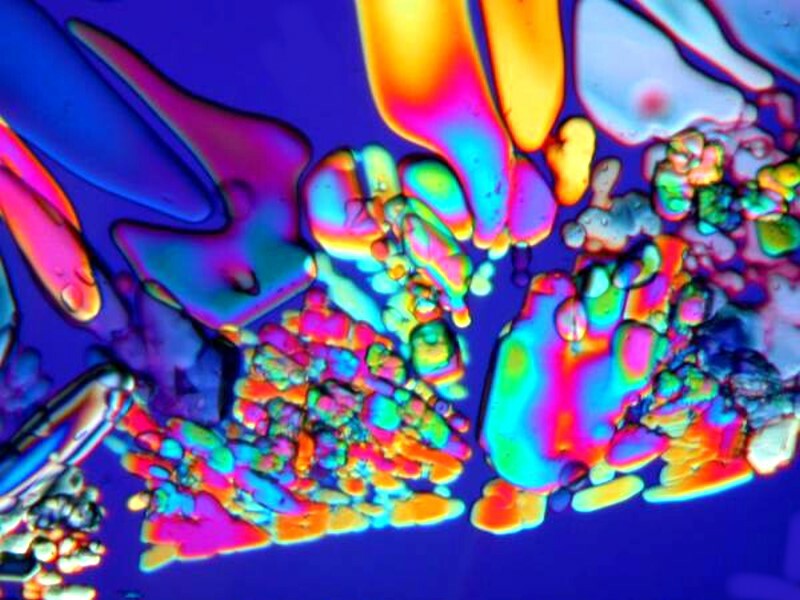
If you look carefully at the first of these three images, you will see a black ring encircling the crystals. This is what makes Magnesium sulfate a bit of a challenge; it is deliquescent and this black line is outlining a drop of water. In other words, the material hasnít yet fully crystallized. You need to get your images quickly if crystallization isnít complete, otherwise the formations will change from minute to minute. This also accounts for the slightly blurry appearance of the image.
When mixed, the results are quite unpredictable as is evident in the image below where I added a drop of the stain Orange G.
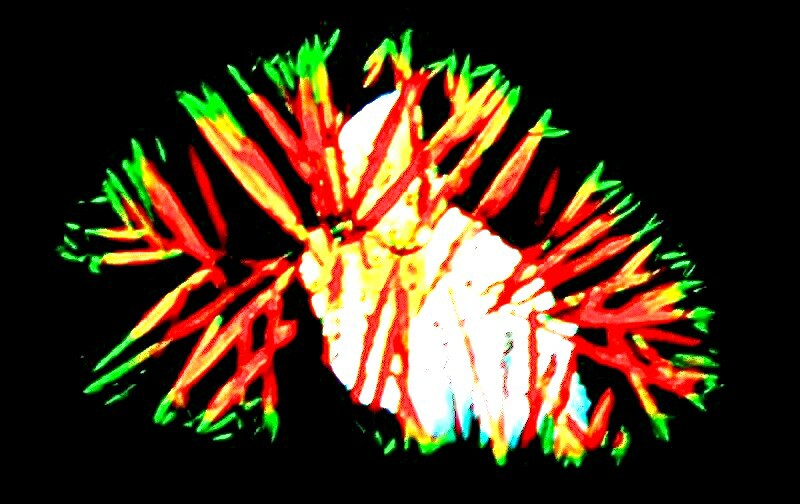
Another highly unpredictable substance is pseudoephedrine which conveniently you can get in a dilute solution and put right onto a slide to dry. Itís usually used to clear a stuffy nose and sinuses. One feature that the 3 images below have in common is a kind of sweeping character that suggest motion.

This second one is cosmic in character suggesting some strange elongated nebula.

This third one is perhaps my favorite of the pseudoephedrine group and that is perhaps because it is combined with a drop of toothache medicine which produces what looks like some lovely, alien bird.

And since weíre talking about Orange G, here is another Orange G and toothache medication combination which I quite like both in terms of color and texture.
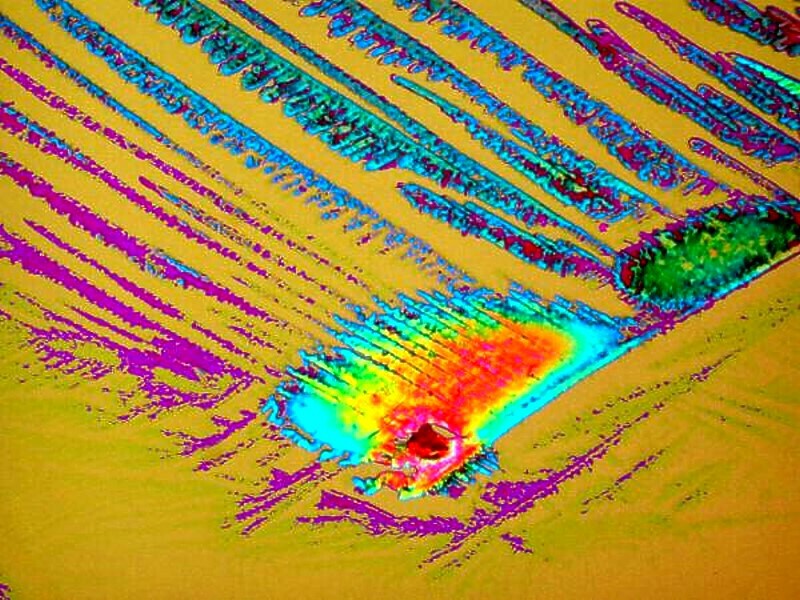
Now, letís look at couple of examples of Orange G combined with Rochelle salts (Potassium Sodium Tartrate tetrahydrate). In the early 19th Century it was used to exhibit piezoelectric effects (and for those of you under 30, no, it has nothing to do with pizza). It is deliquescent which means that it can change while your observing it. So, if you find a crystal pattern you like, you need to have your camera ready to go in order to capture the shot you want. This first image is very colorful and quite feathery.

And with just a light turn of the analyzer in the polarizing system, there is a marvelous shift in the colors.

If we now introduce an antibiotic with a drop of Orange G, we again get some vivid and sweeping forms. The antibiotic in question is Cephalexin.
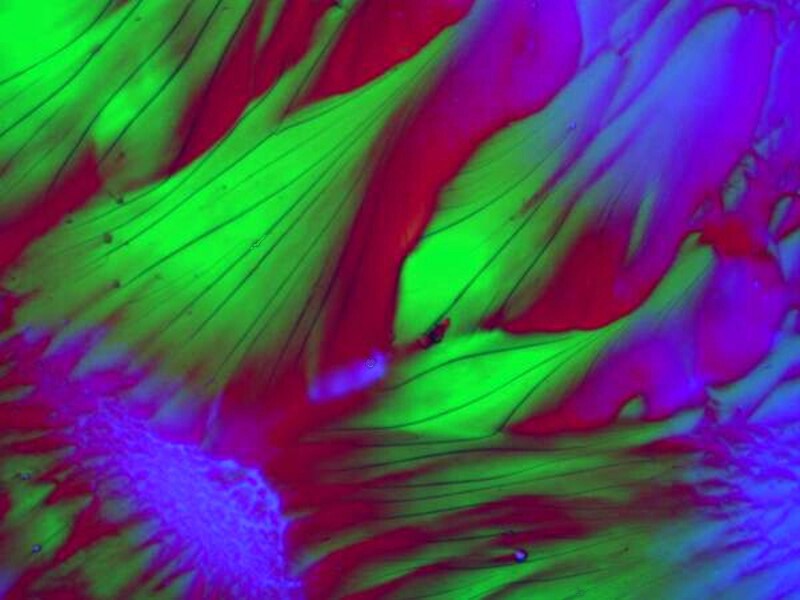
And, at another location on the same slide, a radically different kind of formation.

Copper acetate is another substance that can be counted on to frustrate your attempts at prediction because it is capable of taking on so many different forms, especially when mixed with other substances. The first example is of a disk form and one might at first think that some Ascorbic acid somehow snuck in but, no, itís just copper acetate. And, indeed, most of the colorations are coppery.
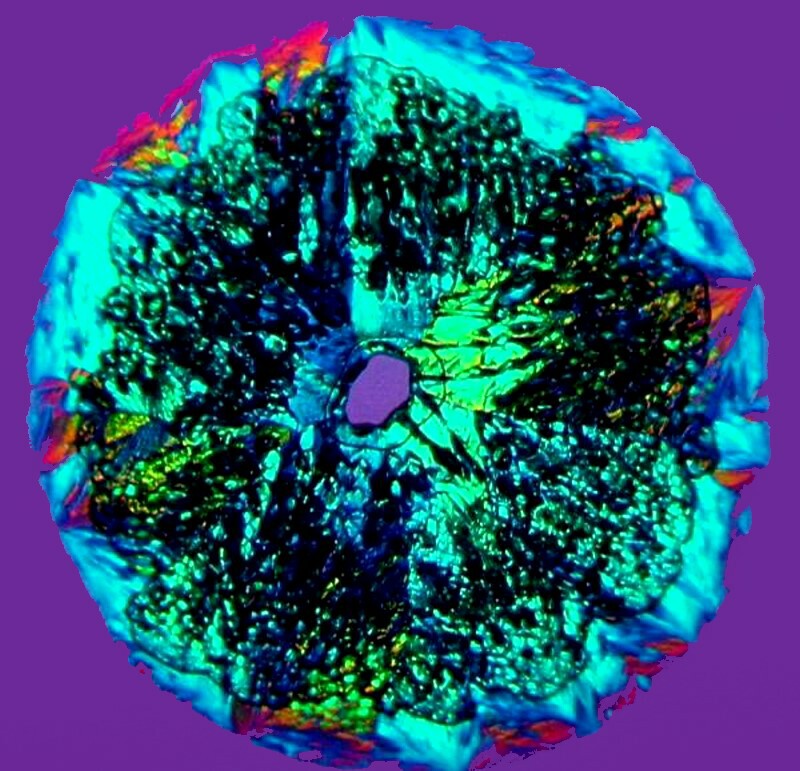
However, if we add a drop of Orange G, we get feathers or perhaps palm fronds.

Now, if we go exploring around the slide, we can even find some very small forms that are geometric and quite pleasing to the eye.
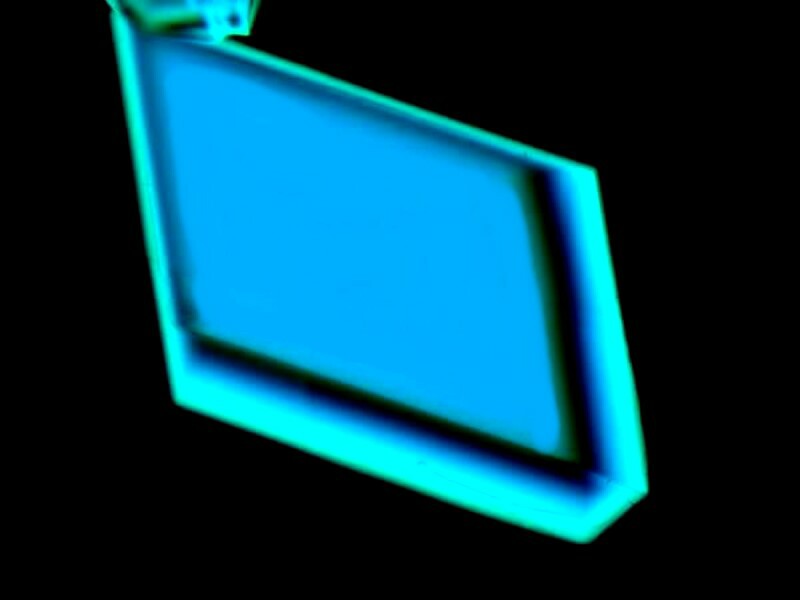

Earlier, we looked at an image that was a mixture of codeine and toothache medicine. Now, letís look at one that is just codeine sulfate.

A minute ago, we encountered some geometric forms in copper acetate, now letís look at some splendid ones produce by IKI (pronounced ICKY). Itís really Lugolís solution or Iodine and Potassium iodide. I find these forms especially appealing and perhaps that dates back from 63 years when I was a mathematics major at university. One can experiment with adding a drop of different stains to the Lugolís or just plain tincture of iodine and getting some different colors.
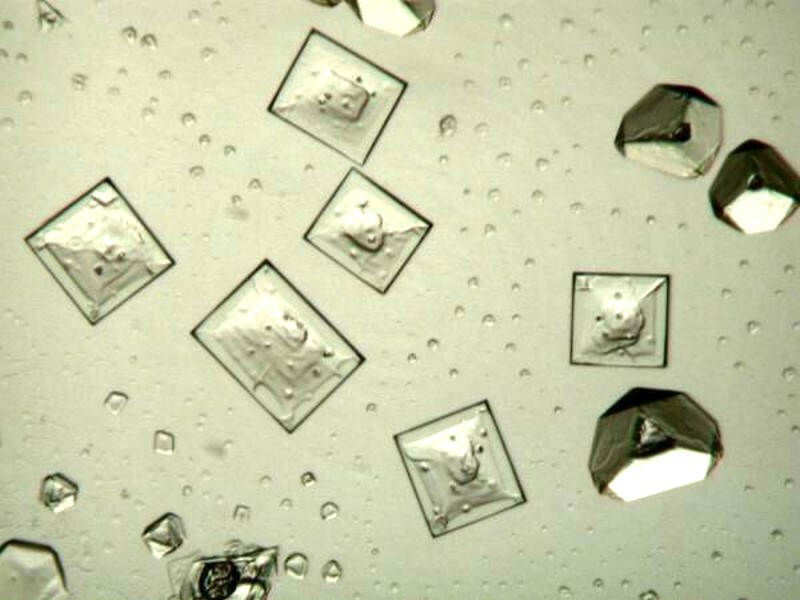
Sometimes, ordinary substances that we have around the house can surprise us and produce some intriguing crystals. For example, I found that hand sanitizer can produce fascinating forms. And, below is an example of a drop of household glue on a slide. Unfortunately, I no longer remember which kind of glue, it was, but thatís O.K., it gives me an opportunity to experiment with several different kinds and see what I find.
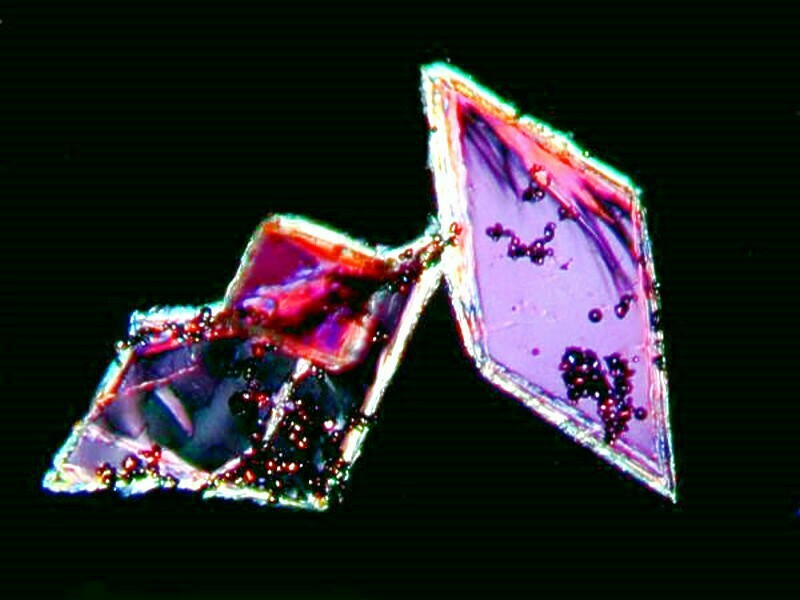
On occasion, just a drop of a single stain can produced nice results. Here is an example with Malachite Green. I canít decide whether itís jagged plant sprigs or green and orange lightning from the planet Ejafjallajokull.
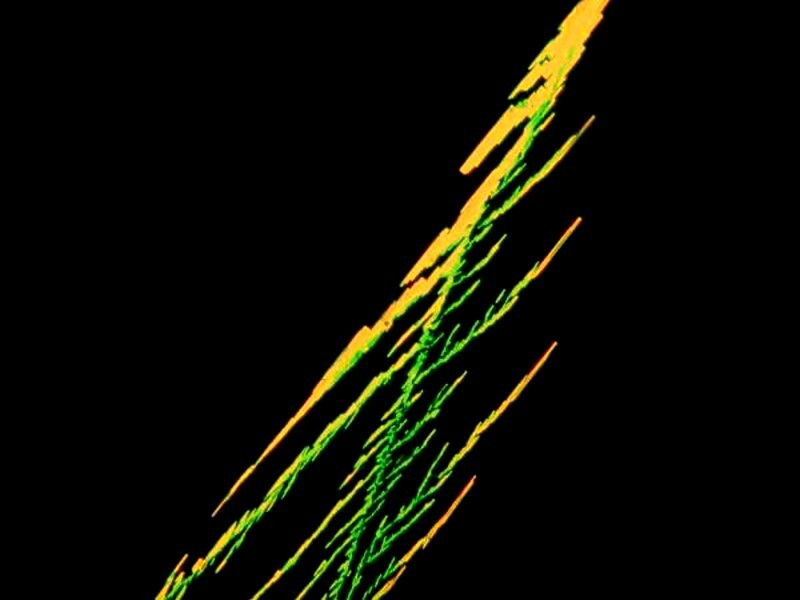
Speaking of alien landscapes, this image of Menthol crystals would certainly qualify in my eyes. Or then again, it might be growths on the back of a bizarre parasite or perhaps red lobsters on the sea bed praying to the sun god.

Alternately, crystals of Sodium acetate might in this case suggest a lava flow into the sea.
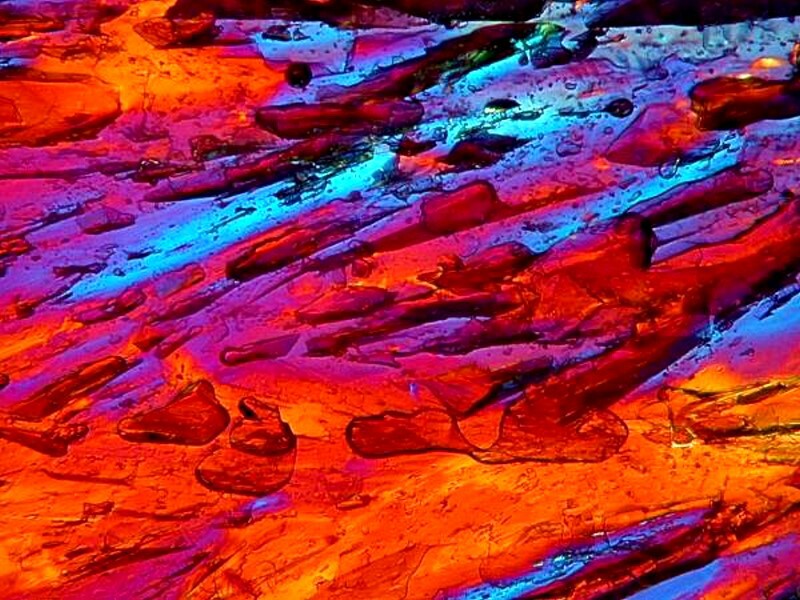
Hereís one for the film photographer who remembers using Sodium thiocyanate. What fascinates me here is that in each of the granules there is the multi-colored ringing in the crystal.
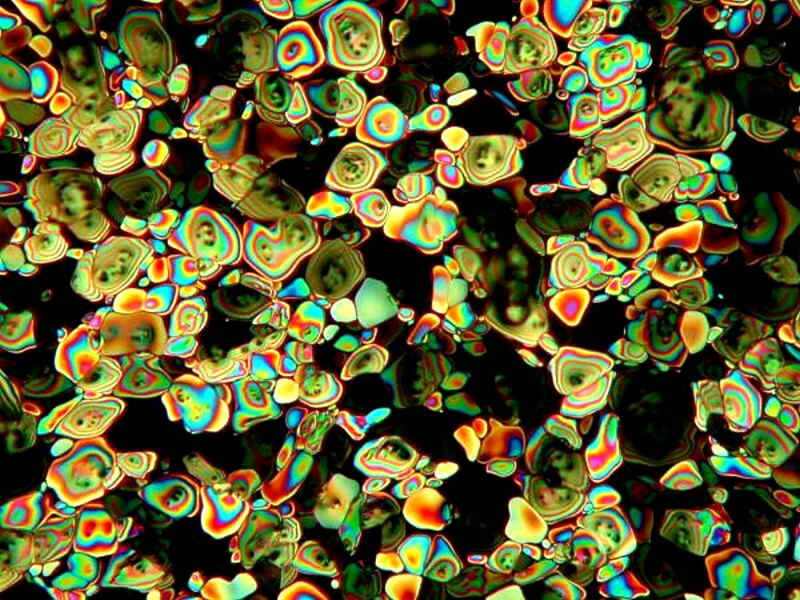
Urea is a substance that produces many types of different crystals. It has a wide variety of uses. The majority of that which is manufactured is used in fertilizers, but there are some medical applications as well. It also has a number of uses which some people might find disconcerting. It is the main ingredient in some hair removing produces; it is a browning agent for pretzels, is used in dish soaps, some skin creams and shampoos, and is often used in tooth whitening products. That being said, here is one image of a crystal formed from a drop of urea on a slide.
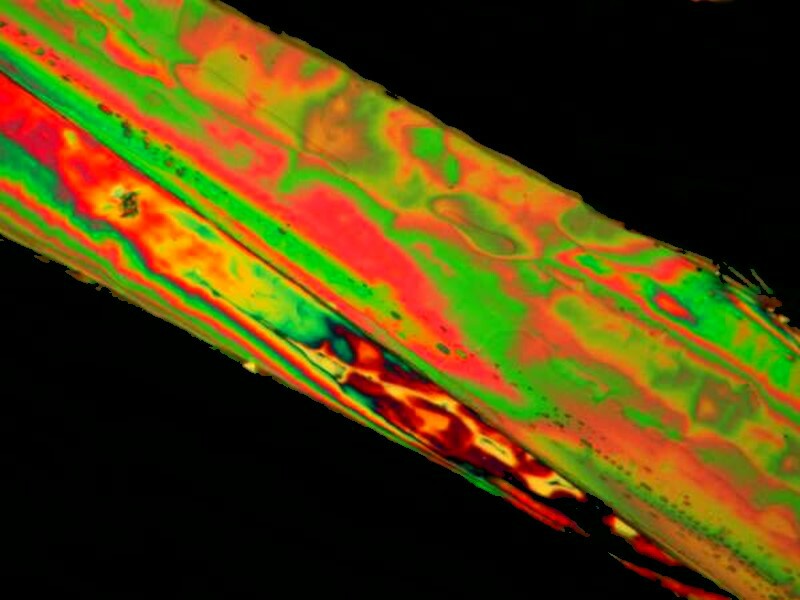
I would like to look at one more Cephalexin and Orange G image before concluding with some Alkaseltzer images. This image perhaps suggests the inner aspects of an artery or other internal vessel.
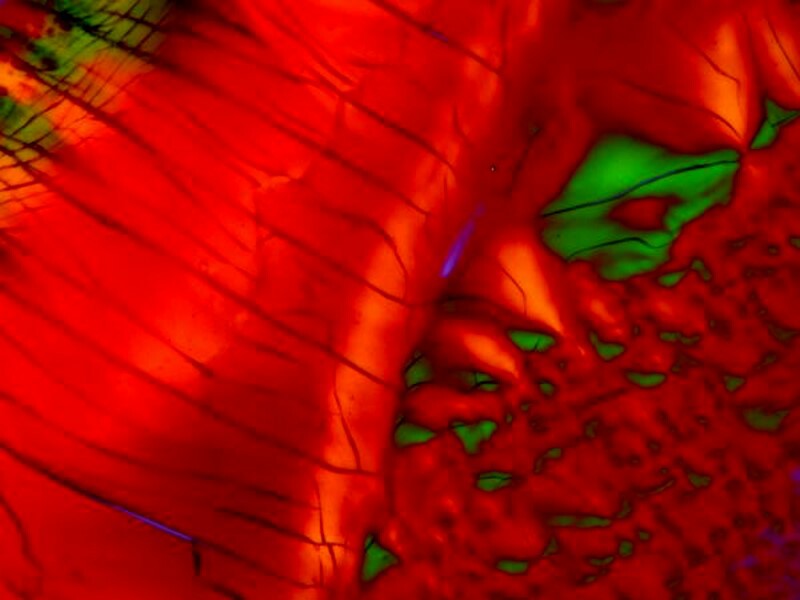
The 5 images below are all of crystals from a generic form of Alkaseltzer. From the first image we might conclude that a bit of Vitamin C was included given the disks forms that are rather typical of Ascorbic acid.
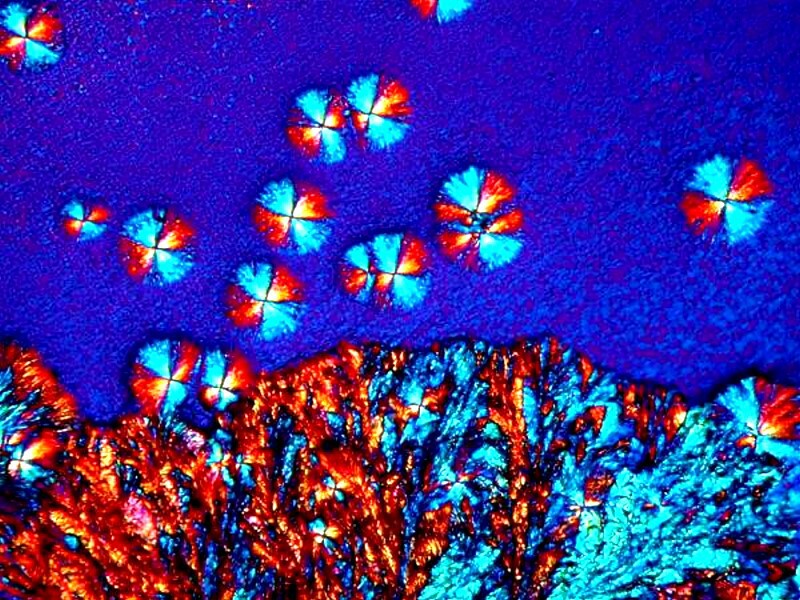
I tend to think of this product as one which produces crystal images which are rather playful, even whimsical. This one has a few Pac-Man figures scampering around gobbling up orange candies.
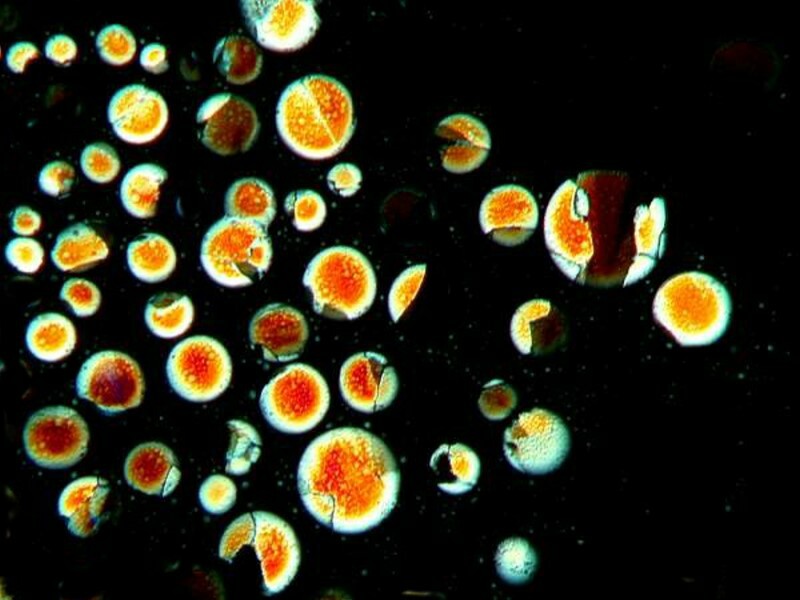
This next image is quite whimsical and for some reason vaguely reminds me of Al Cappís cartoon character the Schmoo, albeit that the ones in this image would have been on a radical diet.
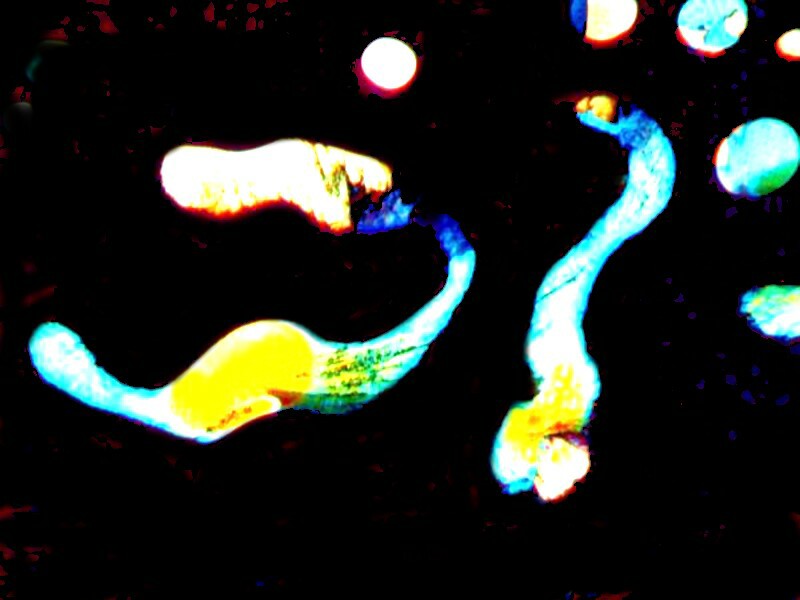
The next one is how I might imaging a surrealist artist depicting a supernova. Itís really quite lovely.
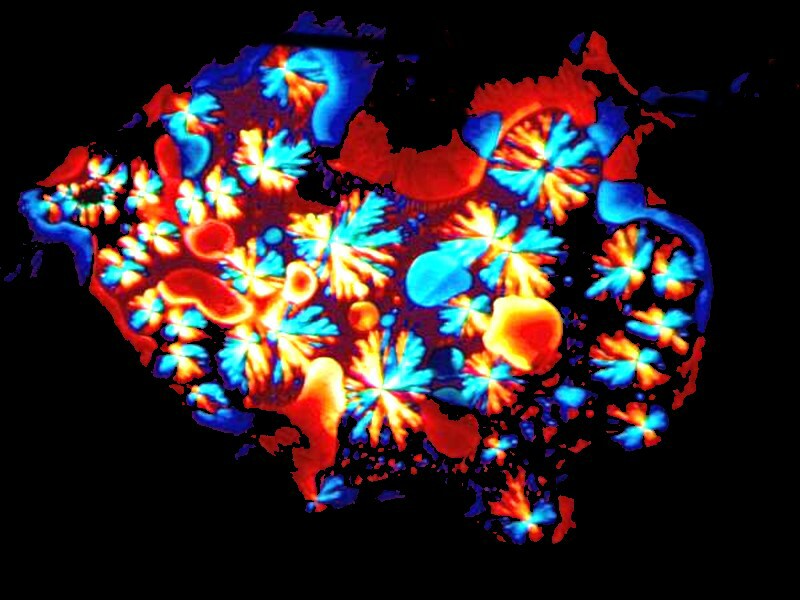
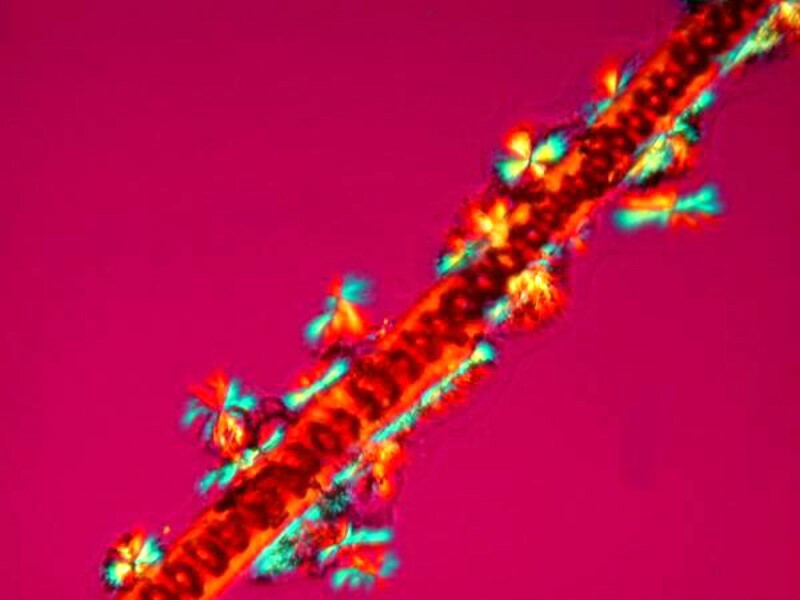
The final one (at last, you gasp) is clearly a strand of Spirogyra with accompanying ectosymbiont space diatoms expelled from the sun.
I hope you have enjoyed the images and will try some experiments of your own with safe household products and share the results with us.
All comments to the author Richard Howey are welcomed.
Editor's note: Visit Richard Howey's new website at http://rhowey.googlepages.com/home where he plans to share aspects of his wide interests.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
© Microscopy UK or their contributors.
Published in the December 2019 edition of Micscape Magazine.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk .