Preparing
and using your own hematein staining solutions using crude
logwood
by Yvan Lindekens,
Belgium

One of those natural dyes that really can’t be missed in microtechnique is hematoxylin. The hematoxylin – eosin staining protocol is used every day, everywhere around the world in histological/pathological labs.
Hematoxylin is derived from the dark red heartwood of the logwood tree Haematoxylum campechianum. You can find some very interesting texts on logwood here and here but a web search will turn up a lot more interesting information. The history of logwood and the logwood trade reads like a first class novel!
A few years after Waldeyer’s unsuccessful trials to use logwood as a histological stain (1863), Böhmer succeeded (1865) in making well-stained slides with hematoxylin. He used a method based on the techniques used at that time by textile dyers: he combined a logwood extract with alum and this became the basic recipe for all known alum hematoxylins.
Hematoxylin in itself isn’t a dye at all: it has to be oxidized (“ripened”) into hematein. The latter, mordanted with a metallic mordant forms a colored lake. The hematein on its turn slowly oxidizes further to form finally trioxyhaematein, which is unusable for staining purposes. So hematoxylin staining solutions are in reality hematein solutions with slowly decreasing –thus unknown - hematein content. Thus the expression “hematoxylin staining solution” is misleading… It is still in use for historical reasons, I suppose.
Some additives known to slow down this oxidation are usually added (glycerin), and the oxidation process is slowed down even more by some ingredients of the solution primarily added for other reasons (alcohol, acetic acid…). Hematoxylin as a dry dye powder is slightly pale salmon-pink, almost colorless, hematein is brown. Hematoxylin is very soluble in ethyl alcohol and in hot water. Hematein only slightly so.
Crude logwood chips are sold as a dye for fabrics. You can buy it online too, for example here and here. I’m sure a web search will turn up a lot more sources. Depending on subspecies and genetic background logwood can contain between 0% and more than 10% hematoxylin.
Of the three samples I bought, only two turned out to be usable. They showed a nice brown-red henna alike color with some metallic green hematein crystals here and there on the surface of the chips. The unusable sample only showed a dull brown.


Crude logwood as it is sold in some shops as a dye for fabrics (L) compared to hematoxylin dry dye powder for microscopy (R)
It’s possible to make an easy to prepare staining solution from crude logwood at home:
Preparation of a hematein staining solution using crude logwood
Saturated solution of potassium alum or ammonium alum in distilled / deionised water: 200 ml
Logwood, finely grinded if possible (it’s very hard!): 10 gm – 20gm
Bring to the boil and let simmer for half an hour. You’ll notice that the mixture has a nice, hibiscus tea or rose bottle tea like smell.
The solution should turn a very dark violet alike color. Let cool down and filter.
To 100 ml of the filtrate, add:
Acetic acid glacial 1 ml, the solution turns a dark red
Glycerin 10 ml
The staining solution is immediately ready to use or not, depending on its hematein content.
If it’s not already usable: add a small drop of diluted hydrogen peroxide (supermarkets: disinfectant, about 3.5% solution), shake and let stand for a few hours.
This staining solution should stay usable for a few weeks.
I suppose an ethyl alcohol extract is usable as a hematoxylin/hematein source, for example to perform Heidenhain’s iron hematoxylin stains but I haven’t tried that (yet).

Comparison of some hematein staining solutions.
From L. to R.: Mayer’s mod. by Lillie prepared with hematoxylin dye powder, above recipe prepared with logwood, Delafield’s prepared with hematoxylin dye powder
Using hematein staining solutions
Hematein stains can be used progressively (stain until the staining result looks okay) or regressively (overstaining followed by a “differentiation” step to remove the hematein-alum lake until an “optimal contrast” is reached between structures that take up or don’t take up the hematein-alum lake). In general regressive stains are superior as they are more selective, thus giving lower background staining levels.
Slides stained with an alum hematein and viewed under the microscope only show a pale red. The color of the hematein-alum lake stain develops only when the slides are brought in a slightly alkaline solution, the so-called “bluing” step. It’s necessary to add a rinse in distilled / deionised water between staining and bluing to prevent the formation of stain deposits on the slide!
The bluing step is also necessary to prevent the stain from fading over time due to the acid content of the staining solution. As tap water is usually slightly alkaline, this can be used to “blue” the slides. Other often used solutions to “blue” hematein stains are:
- 0,1% sodium bicarbonate in distilled /deionised water
- saturated solution of lithium carbonate in distilled / deionised water
- distilled / deionised water with a drop of ammonia added
- Scott’s tap water substitute
- …
A “typical” progressive hematein/eosin Y staining protocol looks something like this:
|
What? |
With? |
How long? |
Why? |
|
Slide in distilled / deionised water |
Distilled / deionised water |
|
- The stain is a solution in water - To prevent the hematein solution to become alkaline, thus compromising it’s shelf life |
|
Stain |
Hematein solution |
Depending on staining solution: between 1 – 20 min |
- To stain nuclei and basophilic structures |
|
Rinse |
Distilled / deionised water |
|
- To remove most of the staining solution - To prevent the formation of stain deposits |
|
“Bluing” |
Tap water or one of the solutions mentioned above |
Until toroughly blue |
- To neutralize any acid left from the staining and differentiation solutions - To obtain a color shift of the hematein-alum lake from pale red to dark blue |
|
Rinse |
Distilled / deionised water |
Several minutes |
- To remove most of the “bluing” solution and any traces left of previously used chemicals |
|
Stain |
0,5% eosin Y in distilled / deionised water |
About one minute |
- To counterstain |
|
Rinse |
Distilled / deionised water |
About one minute |
- To remove most of the eosin solution - To partially differentiate the counterstain |
|
Dehydrate, clear and mount |
|||
And a “typical” regressive hematein/eosin Y staining protocol looks something like this:
|
What? |
With? |
How long? |
Why? |
|
Slide in distilled / deionised water |
Distilled / deionised water |
|
- The stain is a solution in water - To prevent the hematein solution to become alkaline, thus compromising it’s shelf life |
|
Stain |
Hematein solution |
Depending on staining solution: between 1 – 20 min |
- To stain nuclei and basophilic structures |
|
Rinse |
Distilled / deionised water |
|
- To remove most of the staining solution - To prevent the formation of stain deposits |
|
Differentiate |
0,5 % hydrochloric acid in ethyl alcohol 70% |
As long as needed to obtain good contrast |
- To obtain good contrast |
|
Rinse |
Distilled / deionised water |
|
- To remove most of the differentiation solution |
|
“Bluing” |
Tap water or one of the solutions mentioned above |
Until toroughly blue |
- To neutralize any acid left from the staining and differentiation solutions - To obtain a color shift of the hematein-alum lake from pale red to dark blue |
|
Rinse |
Distilled / deionised water |
Several minutes |
- To remove most of the “bluing” solution and any traces left of previously used chemicals |
|
Stain |
0,5% eosin Y in distilled / deionised water |
About one minute |
- To counterstain |
|
Rinse |
Distilled / deionised water |
About one minute |
- To remove most of the eosin solution - To partially differentiate the counterstain |
|
Dehydrate, clear and mount |
|||
Staining results
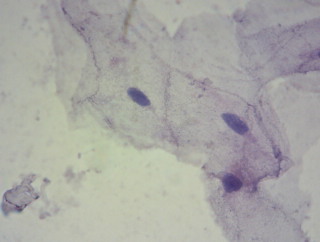
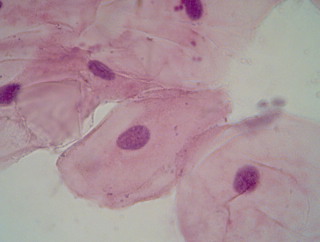
Epithelial cells from the cheek, fixation: dried heat-fixed smear.
Left: Mayer’s acid hemalum mod. by Lillie, progressive, 10 min. at RT, “bluing”: tap water,
Right: the above mentioned staining solution freshly prepared with logwood, progressive, 2hrs. at RT, “bluing”: tap water
Eosin Y counterstain, rinsed in deionised water, dried and mounted in paraffin oil.
The logwood based hematein stain stains a more violet-blue compared to the grey-blue of the Mayer’s. Both show nuclear detail very well.
Zeiss West planachromat 40x, Reichert eyepiece PK 10x. Pictures cropped and resized, no further editing
If you want to experiment using logwood
as a source of hematoxylin/hematein for your own hematein
staining solutions: you can find a lot of hematein recipes
on the Internet, for example
here.