In an earlier article I described some trials I made using near infrared microscopy inspired by the reports of other microscopy enthusiasts, notably Tony Dutton (see ref. 1 and Acknowledgements). By comparing video still image captures taken in transmitted and near IR, I was particularly impressed with how well near IR was suited for visualising and photographing detail in dense insect mounts. I've recently been trying near IR for the macroscopy of prepared slides of larger insect subjects and share these results below. Using this form of lighting involves little cost and certainly worth trying for subjects that may benefit.
The near IR lighting
For greater convenience of using near IR, I retrofitted a bulb in a spare microscope lamp with a near IR emitting LED which has proved useful for both macroscopy and microscopy.
A blown low voltage (6V) tungsten bulb for
my microscope lamp was used to support the LED. This has the benefit of
exploiting the microscope lamp controls to optimise the near IR lighting. The glass envelope of the bulb
was safely removed by scribing all round the base with a triangular
needle file, then a light tap cleanly removed the envelope (taking
appropriate care of hands and eyes in case the glass shattered). Any rough
edges of the lamp base were made safe with emery cloth.
A suitable LED can be soldered onto the filament support struts as shown below. This one is made by Temic and similar designs sell for a few pounds at electronic suppliers.
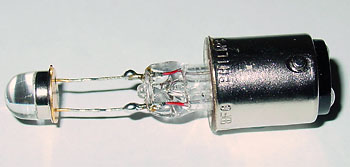
 The
bulb was then installed into a spare microscope lamp, in this case a Russian OI-35 Köhler illumination
lamp (shown on table, image right). A simple battery powered variable intensity
DC supply was made as
described in the earlier article (the typical AC low voltage microscope
lamp supply cannot be used without
modification).
The
bulb was then installed into a spare microscope lamp, in this case a Russian OI-35 Köhler illumination
lamp (shown on table, image right). A simple battery powered variable intensity
DC supply was made as
described in the earlier article (the typical AC low voltage microscope
lamp supply cannot be used without
modification).
Set-up for near IR macroscopy
The set-up used for macroscopy of prepared slides is shown right. The microscope slide is placed directly on the light port. A security video camera (B&W, >560 lines horiz. resolution) was used. Most of the B&W cameras with low light sensitivity should have a good sensitivity to near IR. These are relatively cheap nowadays new.
With a Russian 3.5x objective and 5x eyepiece on my Biolam microscope, the mag. is still far too high to image large areas of insect slides, especially when in my case the camera doesn't image the full visual field and is effectively 'zooming in'. So the set-up shown is useful for very low power microscopy / macroscopy. For near IR microscopy, the lamp is used in its normal way by seating it in the base of the Biolam stand.
The lens is a reversed 50 mm SLR lens and 36 mm extension tube attached via C mount to Nikon bayonet adaptor (ciné lenses with extension tubes can be used if available).
The video output is fed to a portable TV with video input (see cautionary footnote). It's a little strange at first manipulating slides and focusing with no visible sign of light but the TV screen soon becomes your pair of eyes. Image capture was by 640x480 pixel video still with an ageing but hard to beat 'Snappy' capture box (works in Windows XP with available software patch).
Some consumer still digital cameras have a night vision mode (e.g. 'NightShot' mode on higher spec. Sony models) where the IR blocking filter is swung away from the CCD sensor. I'd be interested to hear from readers who try such digicams with this feature on a microscope with near IR lighting.
Image
comparisons below:
Transmitted brightfield: A standard 6V / 15W tungsten
bulb was used with IR blocking filter and video still capture.
Transmitted
near IR: Set-up
and LED as shown above in low visible light which allows the set-up to be safely
used without affecting the near IR image unduly. Images resized from 640x480 with
a small amount of unsharp mask if needed to restore sharpness loss of resizing.
Macroscopy of insect mounts
|
The three prepared
insect slides used for the comparison of transmitted |
|
Macros in transmitted visible light using a bright 35 mm slide viewing table. A very dense mount, left, was chosen, as well as one (right) which shows quite a lot of detail in brightfield. |
|
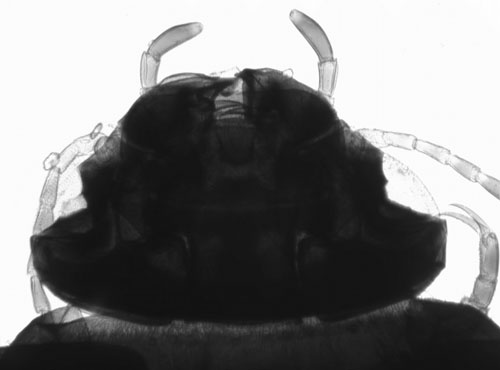
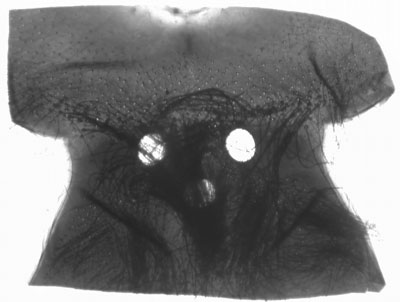
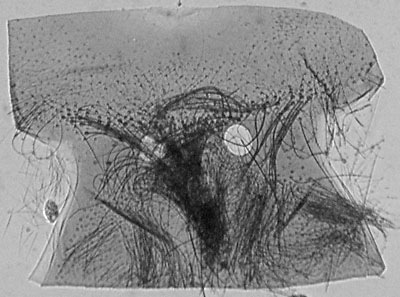
Diving beetle head prepared slide. Transmitted brightfield illumination. This illustrates the typical problem often experienced when imaging whole insect mounts. What little detail can be seen visually through the dense mount requires high illumination levels. The high dynamic range between highlights and shadows is hard to retain when digitally imaged, resulting in burnt out edge definition and little evidence of internal detail. |
|
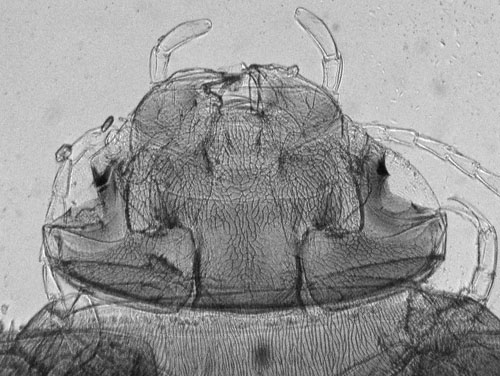
Same subject, transmitted near IR only. This wavelength of light more readily passes through to reveal much more surface and internal detail with a tonal range easier to record. The raw image, (click image above to see it) is very flat, but as no shadow or highlight detail is lost, the raw image responds well to contrast adjustment as performed here using Photoshop Elements. Resolution does decrease when lower wavelengths are used but in the author's view it's not unduly important for this sort of subject. |
|
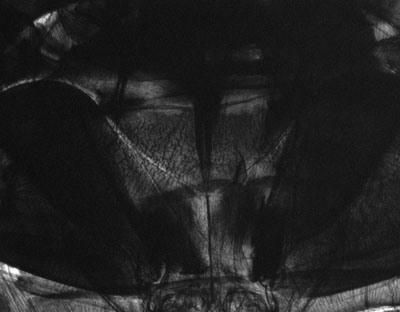
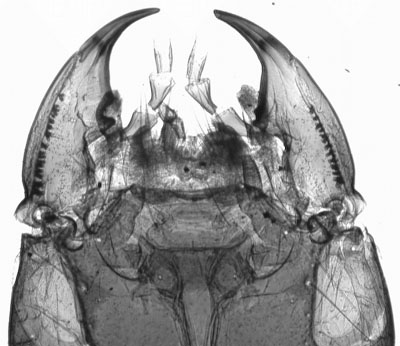
Diving beetle, abdomen, transmitted brightfield. This is nearly opaque in brightfield. |
|
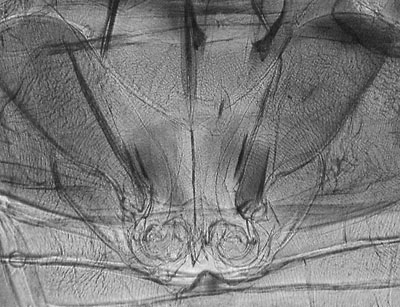
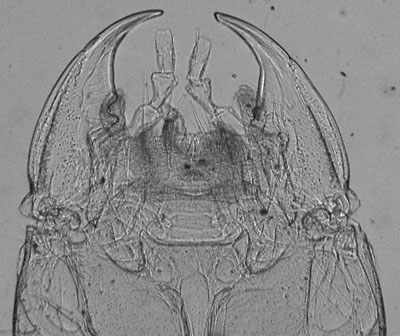
Same subject, in transmitted near IR. Some tonal adjustment, click above image to see raw capture. Considerable detail is revealed. |