|
by Richard L. Howey, Wyoming, USA |
Each image can be clicked with the mouse to view a larger one.
I need to begin by admonishing myself for not following my own advice. I keep telling myself and everybody else who will listen—label everything! Well, the first image is indeed labeled—47A.jpg—but I apparently lost the note (or more likely, never wrote one) identifying this crystal. I suspect—which means, it's a wild guess—that it's from a slide of sodium bicarbonate (ordinary baking soda). I quite like it because it looks like a miniature glass tree to me.
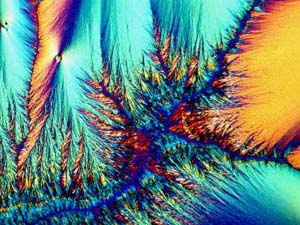
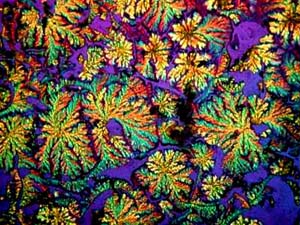
The next three images are all from
one slide of Ascorbic acid (Vitamin C). I should mention here that
all of the images in this article utilized polarized light, although not
always with fully crossed polars. These three images show the wide
range of variation of crystal types which can occur with a single compound.
These variations can be a consequence of a multitude of factors: temperature,
rate of evaporation, solvents, impurities, etc. In cases such as
these, it is not feasible to talk about a "typical" crystal type; however,
there are, of course, other compounds that crystallize out in a quite regular,
consistent, and "well-behaved" manner. As I look at these three images,
I am reminded of some others which I took of ascorbic acid which, because
they were among my first, impressed themselves upon me as "typical" and
I'll include some examples later.
 |
 |
 |
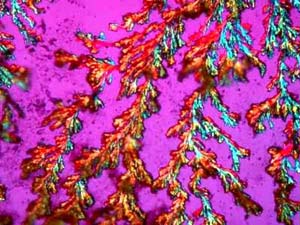
The next image (Bicarb3) demonstrates that a very ordinary household product, in this case, baking soda, can produce wonderful results (in addition to relieving indigestion. I like this image, because it reminds me of a strange metallic ground plant that is rather like a cross between a lichen and a liverwort.
However, again, even such an "ordinary" thing as baking soda can produce some real surprises. Another slide showed radically different forms and, in this case, I was rather reminded of a Navajo tapestry with its bright colors and feather like patterns against a background that emulates white sandstone.
In previous articles, I have mentioned the biological stain, Orange G, which is an important contrast stain and does have some fairly typical patterns of crystallization. However, the micrograph which I have selected here is anything but typical and, in my imagination, conjures images of cosmic gas clouds.
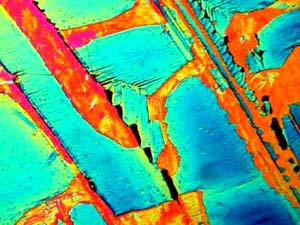
In the two images below of Adipic acid,
you will notice series of slats or elongated "plates" that are fused together
in such a manner that the differences in angle and densities contribute
to the wide variation in color.
 |
 |
What follows are not exotic scrapings
of tartar from my teeth, in spite of the labeling. The next six images
I own to a painful experience—a toothache in the middle of the night.
Before I could get to the dentist to get some antibiotics and painkillers,
I dosed my tooth with a small bottle of liquid toothache medication which
fortunately I had on hand. After the dentist finally got finished,
I still had a bit of the toothache medicine left and made up a slide.
The medicine has benzocaine in it—a topical anesthetic—some dyes (why I
don't know—I don't think the "proper" color is going to be a soothing factor
in the middle of a toothache), a volatile solvent, and various "inert"
ingredients. (Another puzzle—why add extra stuff that doesn't do
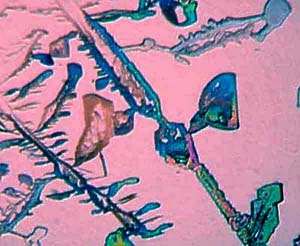
anything?) As you can see in Image 1528, the polars were not completely
crossed and the result is a pink background with delicate pastel hues emanating
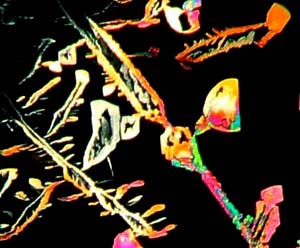
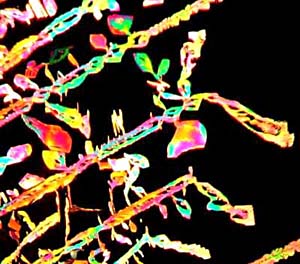
from the crystals. Images 1534, 1536, 1538 and 1541 are from different
portions of the same slide and demonstrate the remarkable variation in
crystalline form and texture which a compound can produce. Some of
these effects are, no doubt, due to those inert ingredients, so they weren't
totally useless after all, although I seriously doubt that the manufacturers
were thinking of us amateur microscopists when they dumped this stuff in
their elixirs. Images 1529, 1534, and 1536 have a certain whimsical
quality that reminds me of some of the paintings of the Swiss artist, Paul
Klee, who often gave his creations delightfully playful titles, such as,
"The Twittering Machine."
1528 |
1529 |

1534 |
1536 |

1538 |

1541 |
Perhaps, that's what I should do, go back and rename all my images using imaginative titles and then "Crystals_tooth1541" could become "The Pain of the Toothache from Hell".
The Ferric ammonium sulfate image surprised me most pleasantly by the combination of the subtle colors and forms suggesting a view through a kaleidoscope.
I like each of the three images of
Potassium phosphate for quite different reasons. The K_phosphate
image might be seen as a snapshot of some subatomic event occurring in
the deep magnetic recesses of a giant particle accelerator and K_phosphate
2494, an image of the same event a nanosecond later. But, of this
series, K_phosphate 2492 is probably my favorite, for it suggests a crystalline
butterfly in an alien landscape with three blades of grass.
 K_phosphate |

K_phosphate-2494 |

K_phosphate-2492 |
Sometimes quite beautiful effects can
be achieved by mixing certain substances. [CAUTION! Always be sure you
know (not just guess) what the reaction of the compounds will be so that
you don't end up producing a poisonous gas or an explosive substance.]
I already mentioned that some chemicals can produce surprisingly different
results from one slide to the next. We already saw one image of sodium
bicarbonate crystallization (Image Bicarb3) and how different it is from
the image on another slide (Image Na_Bicarb1). However, the
next five images are a nice demonstration of a successful mixing of two
chemical which produce some unusual and striking results. You will
notice that in these five images, the general crystal patterns resemble
that of Image Bicarb3 much more than that of Na_Bicarb1. Sodium silicate
(NaSiO4), which I added to the sodium carbonate, is popularly know as "water
glass" and used to be used as an egg preservative. Sometimes, it
can still be found in a local drugstore. I have mixed it with a number
of substances and with varying degrees of interesting results. It
tends, as it dried, to produce a cracked surface, which can result in both
fascinating and dismal effects. It should be handled with some care,
since it is an irritant.

2496 |

2497 |
2498 |

2499 |
2500 |
A technique frequently used and discussed
as a means of producing especially fine examples of crystallization is
that of "melts". This involved placing a bit of a compound on a slide
and heating it carefully until it melts and then placing a clean cover
glass over the crystals. I only recently tried this method and with
only 3 different chemicals. I was making some micro-pipets and decided
to try a few crystal melt slides as well while I had my alcohol lamp out.
I made 2 slides of each compound and the results were dismal except for
an ascorbic acid slide to which I had added a small drop of water before
heating. I didn't like using an open flame so, in future, I will
attempt making such "melts" using a small electric laboratory hot plate
which I have. The great advantage of this device is that it has a
calibrated dial that one can set for different temperatures and some
crystals produce dramatically different crystallization patterns at different
temperatures—copper sulfate, for example—so one should keep careful notes.
The two images of melts below are ones which I took from slides which my
colleague, friend, and adviser extraordinaire on things microscopical,
generously gave me, Mr. Nik Berrong, owner of Rocky Mountain Microscope
Corporation.
'Urea_Melt1' |

'Vanillin_Melt1' |
Finally, I include three images of
Vitamin C. These were prepared by dissolving a commercial Vitamin
C tablet (with all its inert goodies) in water and letting it evaporate
in air without any heating of the slides. (I just took some additional
pictures using chemically "pure" ascorbic acid, but I'll save those for
another gallery.) Image Vitamin C-2481 shows a wonderful pastel swirl
that seems to appear fairly frequently in Vitamin C preparations.
I included a black and white image as well to show how sometimes such an
image can heighten contrast and reveal detail not visible in a color image.
The final image, I include because the double crystal conjures for
me a vision from above of two heavily feathered dervishes whirling away
in some odd pastel world.

2481 |

2482bw |
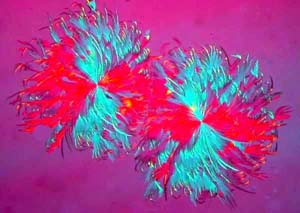
2483 |
All comments to the author
Richard
Howey are welcomed.
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK