

Article by Alan Brinkworth & Maurice Smith - UK Amateurs


Article by Alan Brinkworth & Maurice Smith - UK Amateurs
Note: Methods shown in this article are unsuitable for unsupervised children!
Amateur Microscopy as a hobby, interest, and amateur science pursuit has probably been going on for well over 150 years. Many of the advances made over this time have come about because of the unceasing experimentation and innovation of the enthusiasts keeping the subject alive. One of the main aspects of looking at our world through the microscope lies in the preparation of material for study. After all, without specimen slides, what would we look at with our optical instruments?
Traditionally, only a few substances have proven suitable for 'mounting' specimens: Canada Balsam (a time-honoured natural resin), Glycerin (good for starting out), Numount (as a modern substitute for Balsam and available from Brunel Microscopes and Northern Biological Supplies - follow 'Shop' links), and Fructose (recently re-introduced and advocated by us as an easy non-toxic way of mounting a wide range of subject material).
Each mountant has its own inherent set of advantages and disadvantages. Canada Balsam, for example, has a long drying time before the slide is stable and fixed; although over the years, it has proven to be one of the most stable substances for keeping the mounted material in a good condition. In recent times, the supply of specimen-mounting material has become more difficult, with only a few small businesses making these products available to amateurs. In some countries, the use of solvents and chemicals used for practising microscopy in a hands-on sense, has become subjected to stricter control in a new wave of worry about chemical hazards... making acquisition almost impossible.
As one of the people active at Microscopy-UK in advocating Amateur Microscopy for all, I was delighted to receive a letter along with some slides from my good friend and fellow amateur, Alan Brinkworth, which spoke of a novel new way of mounting material for microscopical study. Like many new ideas, it is often difficult to track down the first person who tried experimenting with new techniques and materials, but in the world of Amateur Microscopy, everyone is prepared to share anything new with others. Anyway, here is is the letter - which Alan has allowed me to publish along with his work. If you decide to try what Alan and other amateurs have done, why not drop us a line and let us know how you get on.
Enclosed are a couple of slides that I produced using Loctite Glass Bond (approximately 2.69 UK pounds per tube from hardware stores and D.I.Y. shops). I thought you would like to see the end results.
The first one, "whiskers", was produced from my own fair chin, dry shaved (it hurts!). The hair was sprinkled onto a very thin smear of Vaseline on a slide. It was then washed in isopropanol to remove any grease and a small blob of Glass Bond was placed onto the slide. A cover slip was placed onto the Glass Bond and pressed down in an attempt to achieve a thin layer of mountant.
As I was doing this at night, I was confounded for a source of UV light (as you are probably aware, it is the Ultra-violet light in sunlight that 'cures' the Glass Bond and sets it). However, it occurred to me that a 12 volt halogen lamp emits UV and this proved correct. I held the slide about 3 inches from a bare halogen light (a lamp without a glass shield) for about 4 minutes and it cured the adhesive. There is a lesson to be learnt here: do not sit too close to halogen lamps, they emit quite a high proportion of UV light and this, as we all know, can be fairly dangerous.
Hair is birefringent - that is - it looks good between crossed polars.
The second slide is of tulip pollen stained with safranin. It was produced in the same way as the whiskers slide with the exception that the curing was done in daylight.
This is a very easy mountant to use but do remember not to sit in bright sunlight when you are applying Glass Bond because it goes off VERY quickly. Any excess mountant around the edge of the cover slip can be easily scrapped off. I then clean up the slide with a small amount of isopropanol.
How these slides survive over long periods of time, I have no idea. As they say -only time will tell.
I do not claim any originality with this method, it was suggested to me by Peter Bruce of the Postal Microscopical Society when I met him at the Northamptonshire Natural History Societies Microscopy Sections, Annual Meeting, this year.
I hope this is of interest to you and anyone else looking for an easy way of producing slides.
regards, Alan Brinkworth
 Great stuff, Alan. Well done. We thought
it would be a good idea to put Alan's home-made slides under the
old scope here at Microscopy-UK so all our readers can see the
very good results. Here are the two slides just as Alan posted
them to us. I know our picture of them isn't very good but you
can see he has used round cover slides and has labelled them with
a pen for simplicity. It is a good idea to label or mark any
slide with its content straight away.
Great stuff, Alan. Well done. We thought
it would be a good idea to put Alan's home-made slides under the
old scope here at Microscopy-UK so all our readers can see the
very good results. Here are the two slides just as Alan posted
them to us. I know our picture of them isn't very good but you
can see he has used round cover slides and has labelled them with
a pen for simplicity. It is a good idea to label or mark any
slide with its content straight away.
 Whiskers
This first image of the 'Whiskers'
slide was taken at 25x magnification using crossed polar filters.
You can see hair shafts in both vertical and transverse sections.
Whiskers
This first image of the 'Whiskers'
slide was taken at 25x magnification using crossed polar filters.
You can see hair shafts in both vertical and transverse sections.
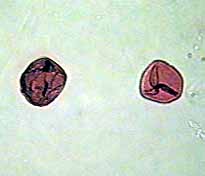 Pollen
Here you can see a couple of the
stained Tulip Pollen. This image was taken at 100x with normal
illumination. Pollen is an interesting area to study!
Pollen
Here you can see a couple of the
stained Tulip Pollen. This image was taken at 100x with normal
illumination. Pollen is an interesting area to study!
Microscopy UK Front
Page
Micscape
Magazine
Article Library
Top
Terms & Glossary
Comments please, to the author Maurice Smith
Footnote
Dr Marvin K. Cook kindly informed us of another mountant:
"Damar varnish is a good medium for mounting specimens.
A word about Damar varnish and its preparation. Singapore Damar
is supposed to be the best. If you need help, I can provide
directions as solubilization is rather slow. Restorers prefer
Damar varnished paintings as it is far easier to remove than
acrylics. Damar in any case is superior to Canada balsam as it
maintains clarity over the years. Canada balsam does yellow in
varying degrees and never completely dries."
Please report any Web problems
or offer general comments to the Micscape Editor,
via the contact on current Micscape Index.
Micscape is the on-line monthly
magazine of the Microscopy UK web
site at Microscopy-UK